Radiation Basics
Radiation is energy. It can come from unstable atoms that undergo radioactive decay, or it can be produced by machines. Radiation travels from its source in the form of energy waves or energized particles.
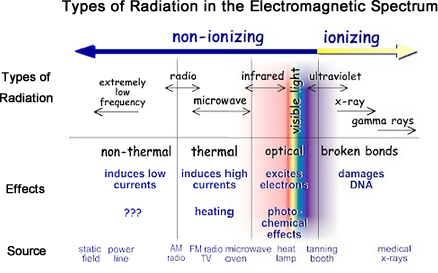
There are two kinds of radiation: ionizing radiation and non-ionizing radiation.
Ionizing radiation has so much energy it can knock electrons out of atoms, a process known as ionization. Ionizing radiation can affect the atoms in living things, so it poses a health risk by damaging tissue and DNA in genes. Ionizing radiation comes from radioactive elements, cosmic particles from outer space and x-ray machines.
Non-ionizing radiation has enough energy to move atoms in a molecule around or cause them to vibrate, but not enough to remove electrons. Examples of this kind of radiation are radio waves, visible light, and microwaves.
EPA’s mission in radiation protection is to protect human health and the environment from the ionizing radiation that comes from human use of radioactive elements. EPA does not regulate the non-ionizing radiation that is emitted by electrical devices such as radio transmitters or cell phones (See: Radiation Information from Other Agencies).
The energy of the radiation shown on the spectrum below increases from left to right as the frequency rises.
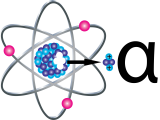
Alpha Particles
Alpha particles (α) are positively charged and made up of two protons and two neutrons from the atom’s nucleus. Alpha particles come from the decay of the heaviest radioactive elements, such as uranium, radium and polonium. Even though alpha particles are very energetic, they are so heavy that they use up their energy over short distances and are unable to travel very far from the atom.
The health effect from exposure to alpha particles depends greatly on how a person is exposed. Alpha particles lack the energy to penetrate even the outer layer of skin, so exposure to the outside of the body is not a major concern. Inside the body, however, they can be very harmful. If alpha-emitters are inhaled, swallowed, or get into the body through a cut, the alpha particles can damage sensitive living tissue. The way these large, heavy particles cause damage makes them more dangerous than other types of radiation. The ionizations they cause are very close together - they can release all their energy in a few cells. This results in more severe damage to cells and DNA.
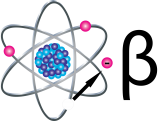
Beta Particles
Beta particles (β) are small, fast-moving particles with a negative electrical charge that are emitted from an atom’s nucleus during radioactive decay. These particles are emitted by certain unstable atoms such as hydrogen-3 (tritium), carbon-14 and strontium-90.
Beta particles are more penetrating than alpha particles, but are less damaging to living tissue and DNA because the ionizations they produce are more widely spaced. They travel farther in air than alpha particles, but can be stopped by a layer of clothing or by a thin layer of a substance such as aluminum. Some beta particles are capable of penetrating the skin and causing damage such as skin burns. However, as with alpha-emitters, beta-emitters are most hazardous when they are inhaled or swallowed.
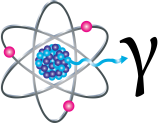
Gamma Rays
Gamma rays (γ) are weightless packets of energy called photons. Unlike alpha and beta particles, which have both energy and mass, gamma rays are pure energy. Gamma rays are similar to visible light, but have much higher energy. Gamma rays are often emitted along with alpha or beta particles during radioactive decay.
Gamma rays are a radiation hazard for the entire body. They can easily penetrate barriers that can stop alpha and beta particles, such as skin and clothing. Gamma rays have so much penetrating power that several inches of a dense material like lead, or even a few feet of concrete may be required to stop them. Gamma rays can pass completely through the human body; as they pass through, they can cause ionizations that damage tissue and DNA.
X-Rays
Because of their use in medicine, almost everyone has heard of x-rays. X-rays are similar to gamma rays in that they are photons of pure energy. X-rays and gamma rays have the same basic properties but come from different parts of the atom. X-rays are emitted from processes outside the nucleus, but gamma rays originate inside the nucleus. They also are generally lower in energy and, therefore less penetrating than gamma rays. X-rays can be produced naturally or by machines using electricity.
Literally thousands of x-ray machines are used daily in medicine. Computerized tomography, commonly known as a CT or CAT scan, uses special x-ray equipment to make detailed images of bones and soft tissue in the body. Medical x-rays are the single largest source of man-made radiation exposure. Learn more about radiation sources and doses. X-rays are also used in industry for inspections and process controls.
There are different but interrelated units for measuring radioactivity and its effects:
- Radioactivity refers to the amount of ionizing radiation released by a material. Whether it emits alpha or beta particles, gamma rays, x-rays, or neutrons, a quantity of radioactive material is expressed in terms of its radioactivity (or simply its activity). This represents how many atoms in the material decay in a given time period. The units of measurement for radioactivity are the curie
 curieThe curie is U.S. unit used to measure radioactivity. One curie is roughly the activity of one gram of Radium-226. Curies are not used to measure radiation dose. The international unit is the Becquerel (Bq). (Ci, U.S. unit) and becquerel
curieThe curie is U.S. unit used to measure radioactivity. One curie is roughly the activity of one gram of Radium-226. Curies are not used to measure radiation dose. The international unit is the Becquerel (Bq). (Ci, U.S. unit) and becquerel becquerelBecquerels are the international unit used to measure radioactivity. One becquerel is the amount of a radioactive material that will undergo one transformation per second. Becquerels are not used to measure radiation dose or radiation exposure. The U.S. unit is the Curie (Ci). (Bq, international unit).
becquerelBecquerels are the international unit used to measure radioactivity. One becquerel is the amount of a radioactive material that will undergo one transformation per second. Becquerels are not used to measure radiation dose or radiation exposure. The U.S. unit is the Curie (Ci). (Bq, international unit).
- Exposure describes the amount of radiation traveling through the air. Many types of radiation monitors measure exposure. The units for exposure are the roentgen (R, U.S. unit) and coulomb/kilogram (C/kg, international unit).
- Absorbed dose describes the amount of radiation absorbed by an object or person. The unit for absorbed dose is the rad
 radThe U.S. unit used to measure absorbed radiation dose (the amount of radiation absorbed by an object or person). The international equivalent is the Gray (Gy). One hundred rads are equal to 1 Gray. (U.S. unit) or the gray
radThe U.S. unit used to measure absorbed radiation dose (the amount of radiation absorbed by an object or person). The international equivalent is the Gray (Gy). One hundred rads are equal to 1 Gray. (U.S. unit) or the gray grayA gray is the international unit used to measure absorbed dose (the amount of radiation absorbed by an object or person). The U.S. unit for absorbed dose is the rad. One gray is equal to 100 rads. (Gy, international unit). One gray is equal to 100 rads.
grayA gray is the international unit used to measure absorbed dose (the amount of radiation absorbed by an object or person). The U.S. unit for absorbed dose is the rad. One gray is equal to 100 rads. (Gy, international unit). One gray is equal to 100 rads.
- Effective dose describes the amount of radiation absorbed by person, adjusted to account for the type of radiation received and the effect on particular organs. The unit used for effective dose is rem
 remThe U.S. unit to measure effective dose. The international unit is sieverts (Sv). (U.S. unit) or sievert
remThe U.S. unit to measure effective dose. The international unit is sieverts (Sv). (U.S. unit) or sievert sievertAn international unit used to measure effective dose. The U.S. unit is rem. (Sv, international unit).
sievertAn international unit used to measure effective dose. The U.S. unit is rem. (Sv, international unit).
One sievert is equal to 100 rems. More commonly, dose is measured in much smaller units: millirems or millisieverts. A millirem![]() milliremThe millirem is the U.S. unit used to measure effective dose. One millirem equals 0.001 rem. The international unit is milliSievert (mSv). (mrem) is one thousandth of a rem. A millisievert is one thousandth of a sievert.
milliremThe millirem is the U.S. unit used to measure effective dose. One millirem equals 0.001 rem. The international unit is milliSievert (mSv). (mrem) is one thousandth of a rem. A millisievert is one thousandth of a sievert.
Use the Radiation Dose Calculator to estimate your yearly dose from sources of ionizing radiation.
Background radiation![]() Background radiationRadiation that is always in the environment. The majority of background radiation occurs naturally and a small fraction comes from man-made elements. is present on Earth at all times. The majority of background radiation occurs naturally from minerals and a small fraction comes from man-made elements. Naturally occurring radioactive minerals in the ground, soil, water and your body produce background radiation, as does cosmic radiation from outer space. There can be large variances in natural background radiation levels from place to place, as well as changes in the same location over time.
Background radiationRadiation that is always in the environment. The majority of background radiation occurs naturally and a small fraction comes from man-made elements. is present on Earth at all times. The majority of background radiation occurs naturally from minerals and a small fraction comes from man-made elements. Naturally occurring radioactive minerals in the ground, soil, water and your body produce background radiation, as does cosmic radiation from outer space. There can be large variances in natural background radiation levels from place to place, as well as changes in the same location over time.
Cosmic Radiation
Cosmic radiation consist of extremely energetic particles that strike the Earth's atmosphere from space. The annual dose of cosmic radiation that people receive depends on elevation. The higher the altitude, the higher the dose. That is why those living in Denver, Colorado (altitude of 5,280 feet) receive a higher annual radiation dose from cosmic radiation than someone living at sea level (altitude of 0 feet). Learn more about cosmic radiation in RadTown USA, EPA's radiation education web area for students and teachers.
Radioactive Materials in the Earth and in Our Bodies
Uranium and thorium naturally found in the earth are called primordial![]() primordialExisting from the beginning of time, naturally occurring. radionuclides
primordialExisting from the beginning of time, naturally occurring. radionuclides![]() radionuclidesRadioactive forms of elements are called radionuclides. Radium-226, Cesium-137, and Strontium-90 are examples of radionuclides. and are the source of terrestrial radiation. Trace amounts of uranium, thorium and their decay products can be found everywhere. Learn more about Radioactive Decay. Terrestrial radiation levels vary by location, but areas with higher concentrations of uranium and thorium in surface soils generally have higher dose levels.
radionuclidesRadioactive forms of elements are called radionuclides. Radium-226, Cesium-137, and Strontium-90 are examples of radionuclides. and are the source of terrestrial radiation. Trace amounts of uranium, thorium and their decay products can be found everywhere. Learn more about Radioactive Decay. Terrestrial radiation levels vary by location, but areas with higher concentrations of uranium and thorium in surface soils generally have higher dose levels.
Traces of radioactive materials can be found in the body, mainly naturally occurring potassium-40. Potassium-40 is found in the food, soil, and water we ingest, allowing it to be absorbed into our bodies.
Man-made Sources
A small fraction of background radiation comes from human activities. Trace amounts of radioactive elements have dispersed in the environment from nuclear weapons tests and accidents like the one at the Chernobyl nuclear power plant in Ukraine. Nuclear reactors emit small amounts of radioactive elements. Radioactive materials used in medicine and even in some consumer products are also a source of small amounts of background radiation. Learn more about radiation and consumer products.