STIR Version 1.0 User's Guide for Pesticide Inhalation Risk
Screening Tool for Inhalation Risk
(November 23, 2010)
Environmental Fate and Effects Division
Office of Pesticide Programs
U.S. Environmental Protection Agency
Washington, D.C.
On this page
- Acknowledgements
- Model Description
- Model History
- When to Use This Model
- Conceptual Model
- Calculation of EECs and Fate Data
- Calculation of Toxicity Endpoints and Ecological Data
- Input Parameters
- Model Results
- Follow-up Actions Based on Model Results
- Discussion of Assumptions and Uncertainties
- References
| Role | Name |
|---|---|
| STIR Developers | Ed Odenkirchen Joseph DeCant |
| STIR Version 1.0 User's Guide and Spreadsheet Tool Project Team Leader |
Joseph DeCant |
| Project Team | Joseph DeCant Christine Hartless |
| QA Manager | Nick Mastrota |
| QA/QC Tester | Catherine Aubee |
| Technical Review Panel | Dirk Young Melissa Panger Gabe Rothman Anita Ullagaddi |
| Beta Testers | Amanda Solliday Valerie Woodard Michael Wagman |
| Management Representative | Mah Shamim |
Thank you to all of the members of the Terrestrial Biology, Fate, and Terrestrial Exposure Technical Teams for their input and assistance during the development of the STIR tool and its associated documentation.
Model Description
The Screening Tool for Inhalation Risk (STIR), estimates inhalation-type exposure based on pesticide-specific information. Physical properties of each chemical, such as molecular weight and vapor pressure, are used to estimate vapor phase exposure. However, this physical chemistry information alone is insufficient for assessing risks due to multiple routes of exposure and chemical specific toxicity. The STIR tool therefore also estimates spray droplet exposure using the application method (e.g., ground versus aerial spray) and rate, and then compares these exposure estimates to avian and mammalian toxicity data. The model uses mammalian-oral, mammalian-inhalation, and avian-oral toxicity values from both registrant-submitted and open literature studies. The model estimates avian-inhalation toxicity values, when not directly available, from mammalian data by applying an adjustment factor representing the difference in lung tissue thickness and surface area between birds and mammals to the relationship between mammalian-oral, mammalian-inhalation, and avian-oral toxicity values to account for differences in avian and mammalian inhalation toxicity (USEPA 2004a).
Model History
Human health risk assessments for field workers and for communities surrounding agricultural areas include an evaluation of inhalation risks to humans. However, existing ecological risk assessment methods for conventional pesticide risks to wildlife do not typically consider this route of exposure. Risk management decisions have not routinely considered risks to wildlife from inhalation of pesticides because recent assessment methods have not allowed for a quantitative evaluation of the potential for pesticide inhalation to contribute to wildlife risk management. The work of Driver et al. (1991), however, suggests that inhalation of pesticides may, in some circumstances, contribute significantly to the potential risks of pesticides to birds following acute exposure. The 2004 FIFRA Scientific Advisory Panel (SAP) (USEPA 2004a) also recommended that risks to avian wildlife should include inhalation as a potential route of exposure.
There are a number of challenges regarding the inclusion and consideration of the inhalation route of exposure in avian and mammalian risk assessments. These include the following:
-
development of an assessment method that is based on existing data from guideline environmental fate and effects studies;
-
use of existing Agency program methods to account for wildlife inhalation exposure and risk;
-
prediction of near-ground air concentrations; and
-
relating pesticide inhalation exposure to oral toxicity data (for avian only).
Currently, there is no standing requirement for avian acute inhalation toxicity data for pesticide registration. Consequently, exposure estimates based on an external inhaled dose cannot be compared directly to effects data from the same route. Typically, the only available avian acute toxicity information is from single oral dose (gavage; acute oral toxicity data) and short-term dietary dose protocols (subacute dietary toxicity data). However, mammalian inhalation toxicity testing is required for human health effects assessments. Therefore, in order to assess the potential risk of inhalation exposure through airborne droplets and vapor phase pesticides, a method is needed that relates mammalian inhalation toxicity information to avian wildlife. In addition, a screening tool is necessary to determine if further analysis and data may be necessary to assess risk through inhalation for both avian and mammalian wildlife. This screening tool provides a means to assess two important questions related to inhalation toxicity:
-
Does a specific chemical have the potential for risk to birds via inhalation exposure once it is applied and subsequently inhaled as either an airborne droplet or vapor phase?
-
Does it also have the potential for risk to mammals via inhalation exposure once it is applied and subsequently inhaled as either an airborne droplet or vapor phase?
When to Use This Model
Science staff within the Environmental Fate and Effects Division (EFED) working on ecological risk assessments will be the primary users of this screening tool. This model will be used as a screening tool in the problem formulation phase of all risk assessments for evaluating potential risk from inhalation for all terrestrial risk assessments by incorporating the toxicity, application rate, and physical properties of a specific chemical. The model will not be used to either quantitatively or qualitatively determine risk; rather the tool will be used to determine whether further data and analysis are necessary to account for potential risks through inhalation.
Conceptual Model
STIR incorporates various pathways by which birds or mammals may be exposed to pesticides. Figure 1 represents a conceptual model for the screening tool. As described in detail later, the screening tool focuses on two major routes of inhalation exposure:
- droplet inhalation immediately after application and
- vapor phase inhalation.
The screening tool incorporates exposure from droplet inhalation by calculating the amount of pesticide sprayed over a defined area based on an assumed duration of time that the pesticide will remain in the air column. The screening tool also addresses the potential volatilization of residues from the treated crop canopy and the soil by calculating the theoretical maximum air concentration at a specified distance above the treated field. Vapor concentrations are equal to the chemical's saturation concentration at 25°C and 1 atmosphere, as determined from the chemical's vapor pressure. These assumptions produce an upper-bound inhalation exposure value. However, the model does not address the transport of particulate bound pesticides.
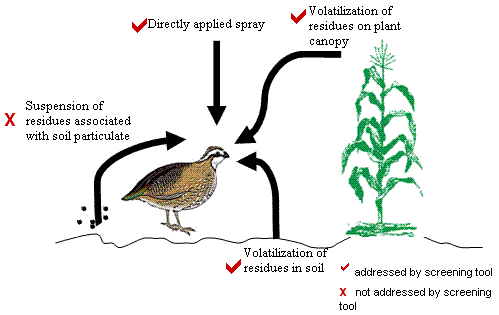 Figure 1
Figure 1
Conceptual model of the various inhalation exposure routes addressed by the STIR model for avian and mammalian wildlife.
Calculation of EECs and Fate Data
The exposure estimates are based on the type of application and how much of a specified chemical is to be applied to a field. If the application type is by spray (either air or ground), the tool estimates both the droplet inhalation and the vapor phase inhalation doses. For non-foliar applications (i.e., granules, seed treatment), the screening tool only calculates the vapor phase inhalation dose.
The method of spray application (either ground or air) and the application rate determine the droplet concentration of the chemical in the air column directly after application based on the height of the spray column (3.3 m for aerial applications, 1 m for ground applications). The inhalation dose depends on this concentration and the duration of direct spray inhalation (1.5 minutes for aerial applications, 30 seconds for ground applications). The size of the spray droplet spectrum that can be inhaled into the lungs is conservatively assumed to be up to 100 µm in diameter. See the section on uncertainties and assumptions for more information regarding these parameters.
The screening tool calculates the theoretical maximum pure product air concentration at standard temperature and pressure based on a chemical's vapor pressure and molecular weight (for saturated air concentration in mg/m3). The model assumes a theoretical maximum pure product air concentration (i.e., saturation at 25°C and 1 atmosphere) as calculated in Equation 1 at a height of six inches above the ground without vegetation. The temperature of 25°C and atmospheric pressure of 1 atmosphere were chosen because vapor pressures are frequently available under these conditions, but the relevance to the actual environment at the time of pesticide application is a cause of uncertainty.
The model also calculates inhalation rates and vapor inhalation doses for birds and mammals. The model conservatively assumes the field active inhalation rate of a bird (Equation 2), which is three-fold greater than the rate of a bird at rest (USEPA, 1993). The maximum 1-hour avian vapor inhalation dose in mg/kg (Equation 3) is a function of the saturated air concentration (Equation 1), the inhalation rate (Equation 2), and the bird weight (assumed to be 0.020 kg, the lowest bird weight modeled in screening assessments). Similarly, the model assumes the field active mammalian inhalation rate (Equation 4), which is three-fold greater than the inhalation rate of a mammal at rest (USEPA, 1993). The maximum 1-hour mammalian vapor inhalation dose in mg/kg (Equation 5) is a function of the saturated air concentration (Equation 1), the mammalian inhalation rate (Equation 4), and the mammal weight (assumed to be 0.015 kg, the lowest mammal weight modeled in screening assessments).
The model calculates the air column concentration of spray droplets after application of the pesticide based on the application rate and spray height (Equation 6). The maximum post-treatment suspended droplet inhalation dose (Equations 7 and 8 for birds and mammals, respectively) occurs when the chemical is applied as a spray treatment. It is a function of the air column concentration (Equation 6), inhalation rate (Equations 2 and 4), duration of direct spray inhalation, and the body weight of the bird or mammal.
Equation 1 - Saturated Air Concentration in mg/m3
Cs = (VP x MW x 1,000,000) / (760 x Vm)
Where:
Cs = the air vapor concentration of a pesticide at saturation in mg/m3
VP = the vapor pressure in mm Hg
MW = the molecular weight in g/mol
Vm = the volume in liters (24.45 L/mol) occupied by 1 mol of a gas at a temperature of 25°C and pressure of 1 atm according to the ideal gas law
1,000,000 = conversion factor in (mg*L)/(g*m3)
760 = pressure at standard pressure and temperature in mm Hg
Equation 2 - Avian Inhalation Rate
IRavian = 284 x (AWavian)0.77 x 60 x A
where:
284 = constant in mL / (kg*min)
IRavian = inhalation rate of assessed bird in cm3/hr
AWavian = body weight of assessed bird in kg (0.02)
60 = hours to minutes conversion
A = activity factor: conversion of resting rate inhalation by multiplying the inhalation rate by a factor of 3 to represent a "field active" inhalation rate
Equation 3 - Maximum Avian Vapor Inhalation Dose
VIDavian = (Cs x IRavian x D) / (1,000,000 x AWavian)
where:
VIDavian = maximum Avian Vapor Inhalation Dose in mg/kg
Cs = saturated air concentration from Equation 1 in mg/m3
IRavian = inhalation rate of assessed bird from Equation 2 in cm3/hr
AWavian = body weight of assessed bird in kg (0.02)
D = duration of exposure, 1 hour
1,000,000 = conversion factor in cm3/m3
Equation 4 - Mammalian Inhalation Rate
IRmammal = 379 x (AWmammal)0.80 x 60 x A
where:
379 = constant in mL / (kg*min)
IRmammal = inhalation rate of assessed mammal in cm3/hr
AWmammal = body weight of assessed mammal in kg (0.015)
60 = hours to minutes conversion
A = activity factor: conversion of resting rate inhalation by multiplying the inhalation rate by a factor of 3 to represent a "field active" inhalation rate
Equation 5 - Maximum Mammalian Vapor Inhalation Dose
VIDmammal = (Cs x IRmammal x D) / (1,000,000 x AWmammal)
where:
VIDmammal = maximum Mammalian Vapor Inhalation Dose in mg/kg
Cs = saturated air concentration from Equation 1 in mg/m3
IRmammal = inhalation rate of assessed mammal from Equation 4 in cm3/hr
AWmammal = body weight of assessed mammal in kg (0.015)
D = duration of exposure, 1 hour
1,000,000 = conversion factor in cm3/m3
Equation 6 - Air Column Concentration after Spray
Cair = AR2 / (H x 100)
where:
Cair = Droplet concentration of the pesticide in the air column in mg/cm3
H = Height of direct spray column (assumed to be 3.3 m for aerial spray or 1 m for ground spray)
100 = conversion factor in cm/m
AR2 = Pesticide application rate in mg/cm2; converted from lbs/acre (AR1) to mg/cm2 according to the equation:
AR2 mg/cm2 = (AR1 lbs/acre x 453.59237 g/lbs x 1000 mg/g) / (40,468,564.2 cm2/acre)
Equation 7 - Avian Spray Droplet Inhalation Dose
SIDavian = (Cair x IRavian x D X Finhaled) / (60 x AWavian)
where:
SIDavian = spray droplet inhalation dose of assessed bird in mg/kg-bw
Cair = Air column concentration after spray in mg a.i./cm3 from Equation 6
IRavian = inhalation rate of assessed bird from Equation 2 in cm3/hr
D = duration of direct spray inhalation (1.5 minutes for aerial and 0.5 minutes for ground applications)
Finhaled = Fraction of spray inhaled
AWavian = body weight of assessed bird in kg (0.02)
60 = hours to minutes conversion
Equation 8 - Mammalian Spray Droplet Inhalation Dose
SIDmammal = (Cair x IRmammal x D x Finhaled) / (60 x AWmammal)
where:
SIDmammal = spray droplet inhalation dose of assessed mammal in mg/kg-bw
Cair = Air column concentration after spray is in mg a.i./cm3 from Equation 6
IRmammal = inhalation rate of assessed mammal from Equation 4 in cm3/hr
D = duration of direct spray inhalation (1.5 minutes for aerial and 0.5 minutes for ground applications)
Finhaled = Fraction of spray inhaled
AWmammal = body weight of assessed mammal in kg (0.015)
60 = hours to minutes conversion
Calculation of Toxicity Endpoints and Ecological Data
Rat acute oral and inhalation toxicity endpoints can be obtained from the "six-pack" of core studies, which are a series of six guideline studies that are submitted to the Registration Division of the Office of Pesticide Programs for formulated and technical grade products of a pesticide. Previous Health Effects Division (HED) or EFED risk assessments may contain this information. If not, these data can be obtained by contacting the appropriate risk manager in the Registration Division for a specific pesticide. Avian acute oral LD50 data may come from either a supplemental or acceptable study that provides the most sensitive toxicity endpoint to be used in the model. These studies may be either registrant-submitted or from open literature identified by the ECOTOXicology database (USEPA, 2007). The decision to use data from a specific study will depend on EFED guidance related to acceptance criteria and the use of open literature (USEPA 2004b, USEPA 2004c). In the absence of these data, STIR cannot be used to evaluate potential risk from inhalation exposure. Depending on the use, these studies should be requested as they are required under the 40 CFR § 158 regulations for most outdoor uses.
The body weight of the tested species must also be obtained. Default body weight assumptions for the laboratory rat, Mallard duck, and Bobwhite quail are provided in STIR. Use of other species (in the case of birds) or studies involving subject animals markedly different from the assumed body weights requires the risk assessor to provide the alternate body weight. These data should be obtained from the study report if possible (time weighted average). Alternatively, referenced body weight values may be obtained from a variety of sources, including USEPA (1993) and the testing laboratory.
The screening tool estimates a bird inhalation LD50 value (mg/kg-bw) by applying the relationship between mammalian oral and inhalation LD50 values to the most sensitive avian oral LD50 value. The rat inhalation LD50 is a function of the rat inhalation LC50 using a modified version of the HED extrapolation method (Equation 9), which is based on the Stokinger and Woodward method of route-to-route extrapolation (Pepelko and Withey, 1985). There are numerous uncertainties related to route-to-route extrapolations, including the inability to metabolize a toxin through inhalation compared to oral exposure and absorption differences across the gut compared to the lung. This method provides a means of avoiding the extrapolation uncertainties by simply converting a concentration based endpoint, (i.e., the median lethal concentration for 50% of the animals tested, LC50) to an equivalent median lethal inhalation dose for 50% of the animals tested (LD50) based on the volume of inhaled air, body weight, animal activity, and absorption efficiency. The conversion factor (CF, Equation 10) is modified for the purposes of this model to account for the inhalation rate of the mammal on an acute basis and uses the default weight assumption of 0.350 kg based on the value used in the Terrestrial Residue EXposure model (T-REX, v1.4.1; Oct. 9, 2008), which is currently used by EFED for terrestrial risk assessments for birds and mammals. The mammalian inhalation LD50 is then adjusted to the body size of a 0.015 kg mammal (Equation 10) based on allometric equations relating the oral LD50 to body weight as applied in the T-REX. STIR uses the ratio of avian to mammalian relative diffusion rates across lung tissue (Qa/Qm; US EPA 2004a) to account for differences between avian and mammalian lung membrane thickness and surface area as they relate to absorption efficiency. STIR then adjusts the estimated bird inhalation LD50 (Equation 11) to the body size of a 0.020 kg bird (Equation 12) based on the allometric equation relating the avian oral LD50 to body weight as applied in the T-REX.
Equation 9 - Conversion of Mammalian Inhalation LC50 to LD50
LD50 = LC50 x Abs x CF x D x A
where:
LD50 in mg/kg-bw
LC50 in mg/L
Abs = 1, absorption is assumed to be 100%
CF = Conversion factor, a L/(hr*kg) factor derived from Equation 5
where CF = [mammalian inhalation rate (cm3/hr) * 0.001 (L/cm3)] / Body Weight (kg).
D = Duration of the inhalation study, usually 4 hours
A = Activity factor where the animal default is 1; this value reflects the rat at rest in the experimental conditions
Equation 10 - Adjusted Mammalian Inhalation LD50
LD50adj = LD50 x (TWmammal / AWmammal) 0.25
where:
LD50adj in mg/kg-bw
AWmammal = Body weight of assessed mammal (0.015 kg rat)
TWmammal = Body weight of tested mammal (e.g. 0.350 kg rat)
All LD50 values are in mg/kg-bw
Equation 11 - Estimated Avian Inhalation LD50
LD50est = (LD50ao x LD50ri) / (3.5 x LD50ro)
where:
LD50 est = estimated avian inhalation LD50
LD50 ao = avian oral LD50
LD50 ri = rat inhalation LD50
LD50 ro = rat oral LD50
All LD50 values are in mg/kg-bw
3.5 = unitless adjustment factor (Qa/Qm; USEPA, 2004a) to account for the higher level of expected toxicant absorption based on surface area and membrane thickness of the avian lung versus the mammalian lung
Equation 12 - Adjusted Avian Inhalation LD50
LD50adj = LD50 x (AWavian / TWavian)(x-1)
where:
AWavian = Body weight of assessed bird (0.02 kg)
TWavian = Body weight of tested bird (e.g. 1.580 kg Mallard duck, 0.178 kg Bobwhite quail)
All LD50 values are in mg/kg-bw
x = Mineau scaling factor for birds1
1EFED default is 1.15; however, the appropriate chemical-specific value should be used when available. The chemical-specific scaling factors can be found in Mineau et al. 1996.
Input Parameters
The STIR tool is implemented in a Microsoft® Excel 2003 spreadsheet. All of the input parameters will be entered in the STIR spreadsheet in the section entitled "Input" except for the fraction of the spray distribution that is inhaled, which will be entered in another section of the spreadsheet. Figure 2 shows a corresponding screenshot that illustrates the layout of this section.
The fraction of inhaled spray parameter will require the use of AgDrift (v2.01; May 24, 2001) and the information provided on the chemical labels. If the label does not specify the droplet size of the spray application, then the STIR default of 90% for the fraction of inhalable spray droplets will be used and the chemical reviewer does not need to adjust STIR. This percentage represents the most conservative spray application scenario, which is the aerosol to very fine distribution. In this distribution, 90% of the particulates are 100 µm or less in diameter. If the droplet size is specified on the label, then the chemical reviewer should use the Droplet Size Distribution (DSD) option in the Tier II assessment tab of AgDrift. After selecting the DSD option, the reviewer should select the droplet distribution of interest on the left of the screen. The reviewer should identify the row in the table on the right that corresponds to "98.12" from the first column that reads, "Average Diameter (µm)". The value from the third column ("Cumulative Volume Fraction") in that row provides the cumulative fraction of the spray distribution that extends up to 98.12 µm in diameter. This value should be entered into cell "M8" of STIR that corresponds to the "fraction of spray inhaled", which is highlighted in yellow.
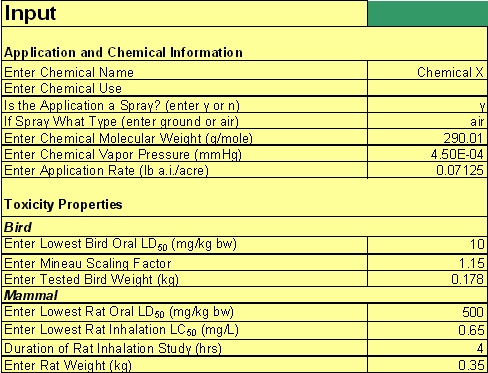 Figure 2
Figure 2
Screenshot of hypothetical STIR.xls data input section.
The effects data to be used will come from studies that were conducted in accordance with three OPPTS Harmonized Test Guidelines (Series 850 Ecological Effects and 870 Mammalian Toxicology): the most sensitive LD50 value from 850.2100 (Avian Acute Oral Toxicity Test) and 870.1100 (Acute Oral Toxicity – Rat), and LC50 value from 870.1300 (4-hour Acute Inhalation Toxicity – Rat).
As stated on the Agency's website (Final Test Guidelines for Pesticides and Toxic Substances), these harmonized test guidelines have been developed for use in the testing of pesticides and toxic substances and indicate the type of test data that must be submitted to the Agency for review under Federal regulations.
Exposure values will be derived from information on the physical characteristics and methods of application of a specific chemical. This information is obtained from the registrant-submitted product chemistry data and pesticide labels. Input parameters include the proposed maximum application rate (lbs a.i./acre), application method (aerial/ground, spray), molecular weight (g/mole), and vapor pressure (mm Hg).
Model Results
The screening tool presents the model results as a ratio of the estimated inhalation exposure "dose" to toxicity (Figure 3). This ratio is then compared to a threshold (0.1) that represents one-tenth of the LD50. This threshold is intended to be protective of all listed and non-listed avian and mammalian species, based on the current acute endangered species level of concern (LOC).
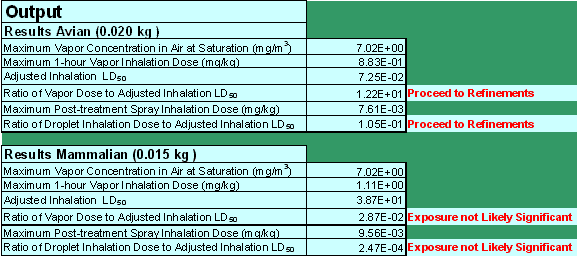 Figure 3
Figure 3
Screenshot of hypothetical STIR.xls data output section.
The calculated ratios of exposure to toxicity represent the two inhalation exposure pathways. These ratios of exposure to toxicity are two separate calculations for both avian and mammalian species. The ratio of the vapor dose to the adjusted avian inhalation LD50 is simply Equation 3 divided by Equation 12. Similarly, the ratio of droplet inhalation dose to the adjusted avian inhalation LD50 is Equation 7 divided by Equation 12. The ratio of the vapor or droplet dose to the adjusted mammalian inhalation LD50 is Equation 5 or 8, respectively, divided by the adjusted mammalian inhalation LD50 in Equation 10. The exposure values that the model estimates are calculated as two separate exposure events and should not be combined into one composite exposure value. Furthermore, the ratios do not quantify the magnitude of risk, and so the magnitude of the output ratios cannot be compared. The ratios simply indicate the potential for risk when compared to the specified threshold of protection.
The reviewer may already have available data that eliminates the need for some of the estimated parameters in STIR. For example, an avian inhalation study may have been submitted to EFED, which provides a definitive inhalation toxicity endpoint. In this case, the chemical reviewer can use the avian inhalation study results and incorporate them into the correct cell in the spreadsheet to calculate the specific ratios.
If the ratios are less than 0.1, the model indicates that the inhalation exposures are not likely significant on an acute basis from inhalation exposure alone and no further action is necessary. If any of these ratios exceed 0.1, the model indicates that the available screening methodology cannot dismiss the potential for significant spray droplet or vapor phase inhalation risk and further analysis is recommended. Further analysis can then be pursued by requesting additional studies based upon the best available science to contribute to more refined approaches that evaluate risk via inhalation exposure. The additional studies as well as the risk assessment methods will be discussed more in the following section.
Follow-up Actions Based on Model Results
The results of the STIR tool do not definitively assess risk from inhalation but rather provide a screening level evaluation of the need for more information to better characterize inhalation risk. Therefore, the chemical reviewer should briefly discuss the STIR tool in the analysis plan of the problem formulation section of the risk assessment. For the problem formulation step of an action, in sections describing the assumptions of exposure pathways of concern, STIR should be referenced and the results discussed therein to explain if inhalation will be considered in the risk assessment proper or not. The results of STIR present the ratio of potential maximal exposure to an assumption of lethal inhalation toxicity. If these ratios are high enough to trigger a concern for this pathway (i.e., ≥ 0.1) then the problem formulation should discuss the pathway as potentially complete and potentially significant. The Problem Formulation should then also discuss appropriate data requirements to inform a more definitive estimation of inhalation risk. The section of the Problem Formulation describing data gaps should reference STIR and the results of STIR to inform and justify any data requested to support a more definitive assessment of inhalation risks.
If the tool suggests that the reviewer "proceed to refinements" for either vapor or direct spray inhalation, the reviewer should request an avian inhalation study. Registrants have submitted this type of study before and have usually adapted the methods outlined in the rat inhalation toxicity study (OPPTS Guideline 870.1300) to avian species. The reviewer can suggest a species to be used based on the species tested for the oral or sub-acute dietary toxicity. If existing data show a particular size class (e.g. bobwhite quail, mallard duck, passerine) to be more sensitive to a specific chemical, the test species in that size class should be suggested.
The data potentially recommended based on the results of STIR and enumerated in the Problem Formulation to support a more definitive inhalation risk assessment by EFED will provide empirically derived toxicity endpoints. A screening level risk assessment for inhalation exposure will incorporate these toxicity endpoints with more refined estimates of exposure. More definitive exposure estimates from spray drift can be obtained through the use of AgDrift. With AgDrift, the chemical reviewer can further evaluate spray drift exposure on the target field as well as the distance of movement off-site. A flux rate coupled with the use of a dispersion model will provide an estimate of exposure for the vapor phase pesticide. More refined approaches to integrate toxicity and exposure may use the Terrestrial Investigation Model (TIM, USEPA 2004a, currently being revised) to derive probabilities of magnitude of avian mortality events. More information on methods for the screening level risk assessment will be provided in future updates to this user's guide as the methods are further developed.
Discussion of Assumptions and Uncertainties
The exposure assumptions are based upon a vapor concentration at saturation at a temperature of 25°C. Temperatures at the time of pesticide applications could differ from 25°C, with higher temperatures resulting in higher vapor pressures. The value of 25°C is advantageous, however, because much vapor pressure data are available at this temperature. In addition, it does not seem to be an unreasonable estimate of an environmentally relevant temperature at the time of pesticide application. This does, however, add uncertainty into the calculations. Future versions of STIR may incorporate scenario-specific temperatures to which the vapor pressure would be adjusted accordingly.
STIR considers the vapor concentration to be at saturation. The assumption that a chemical's vapor pressure represents its partial pressure under a saturated state is inherently based on the definition of vapor pressure and is prescribed to be measured accordingly in OPPTS 830.7950 Product Properties Test Guidelines. The vapor pressure at saturation represents the highest concentration at which the chemical can exist and therefore, represents an upper-bound exposure value and provides a conservative estimate of exposure at the screening level.
The current test guideline for mammalian inhalation toxicity involves test organisms exposed to a pesticide through atomization rather than volatilization. The STIR model's exposure routine that evaluates exposure through inhalation of direct spray droplets is therefore similar to the mammalian toxicity protocol employed for the acute mammal inhalation effects endpoint. However, the vapor phase exposure estimate is not. Exposure to a volatilized chemical will likely involve different absorption rates and reach different loci for absorption as compared to a chemical presented to test organisms in atomized form. These differences in the absorption of a vapor relative to an aerosol may enhance and possibly alter the toxic effects. In the absence of actual vapor phase effects endpoint testing, it is possible that the STIR reliance on aerosol effects endpoints may underestimate risk for organisms inhaling pesticide in the vapor form. However, in the absence of additional vapor phase testing, the current inhalation toxicity guideline represents the best available science to evaluate inhalation toxicity.
The method of deriving avian inhalation toxicity as outlined in this document provides an estimate of inhalation toxicity to avian species based on the current state of knowledge regarding avian respiratory physiology. The 2004 SAP (US EPA, 2004a) listed several uncertainties that remain in any attempt to estimate avian inhalation toxicity:
-
Differences in vascularization of mammalian and avian lungs: may influence overall diffusion rates of xenobiotics
-
Differences in enzymatic activity in lung tissues: may affect rates of chemical formation
These uncertainties represent opportunities for future areas of research to improve the ability of the model to predict inhalation toxicity in the absence of route/species specific data. Once this information becomes available, the model may be updated as needed.
The screening tool is based upon the estimated avian inhalation LD50 for an assumed 0.020 kg bird and a mammalian inhalation LD50 for an assumed 0.015 kg mammal. A 0.020 kg bird is the lowest bird weight modeled in screening assessments; however, questions remain about the ability to draw inferences from this bird weight to other bird weights. The weight of a 0.020 kg bird is used to derive the most conservative inhalation dose by incorporating the weight of the bird into the allometric equation relating inhalation rates to bird weights for non-passerines as described in US EPA, 1993, and Equation 2 in this document. As the bird weight increases, the amount of pesticide that is inhaled increases because the respiratory volume increases. However, the dose per unit body weight decreases and hence the use of the 0.020 kg bird to obtain a conservative dose estimate.
As mentioned in US EPA, 1993, the allometric equation for avian inhalation rates is based on the work of Lasiewski and Calder (1971) that focused on non-passerines and specifically excluded the passerines, which have a somewhat higher metabolic rate. Other authors (Lasiewski and Dawson, 1967; Nagy, 1987) have shown that the metabolic rate for passerines is 1.65 times greater for basal metabolic rate and 1.85 times greater for free living metabolic rate compared to non-passerines. However, STIR continues to employ this allometric equation to scale to a 0.020 kg bird that could represent either a passerine or non-passerine bird. As a consequence, STIR may underestimate the inhalation dose from both vapor phase and droplet exposure to passerine species. The model still provides a conservative estimate for avian species even though it focuses on non-passerine inhalation rates by incorporating other conservative aspects of inhalation risk such as the ability of the bird to inhale pesticide particles up to 100 µm in diameter, saturated air concentrations based on vapor pressure, and the use of the 0.020 kg bird weight.
Toxicity data (oral LD50) are derived from the most sensitive species tested in either an acceptable or supplemental study that has been selected by the EFED reviewer. This species will likely be larger than a 0.020 kg bird or a 0.015 kg mammal. One could assume that the toxicity of the specific pesticide will be constant across bird or mammal sizes (i.e., the inhalation LD50 is not a function of size). However, research has shown both the importance of body weight in assessing toxicity and the relevance of allometric equations to adjusting toxicity values for weight (Mineau et al., 1996; Sample and Arenal, 1999). For this reason, current EFED models such as T-REX incorporate allometric equations to adjust oral toxicity values according to body weight.
Despite the apparent need, the available research has not specifically addressed allometric equations that relate bird and mammal weight to inhalation toxicity. There is a lack of information from the open literature on inhalation toxicity of semi-volatile and volatile pesticides to birds and mammals of different sizes. The Agency also has little information on inhalation toxicity across varying weights to mammals and especially birds in general. The current use of allometric equations reviewed above represents the best available science that can be used in the context of inhalation toxicity. This model therefore employs the same allometric adjustment to the LD50 to account for differences in the size of the bird or mammal in order to obtain an adjusted LD50 of a 0.020 kg bird or 0.015 kg mammal. In addition, the model accounts for differences in respiratory volume by applying the allometric equation relating body weight to inhalation rate to obtain a weight adjusted volume inhaled per unit time. By adjusting both the LD50 and the inhalation rate to a 0.020 kg bird and a 0.015 kg mammal, this tool provides a conservative comparison of exposure to toxicity based on the current state of knowledge.
Another assumption is the ability of the bird or mammal to inhale particles of 100 µm in diameter or less of the direct spray droplet distribution immediately after application of the pesticide. Previous discussions at the 2004 SAP (US EPA, 2004a) provided insight on the size of particles that a bird is able to inhale and respire. Inhalable particles are those that pass through the nares and enter the upper respiratory tract, but are not able to enter the lungs or air sacs, whereas respirable particles are those that can enter the lungs. The 2004 SAP (US EPA, 2004a) identified 7 µm to be the largest particle size able to enter the lungs of a bird. However, it also stated that "While this [7 µm particle size] is certainly not likely to be the maximum, prediction of the maximum particle size would undoubtedly be complex, leaving 7 µm as the most plausible estimate." The review of available literature by the 2004 SAP (US EPA, 2004a) identified limitations with the data and suggested that larger particle sizes may be able to enter the respiratory system of a bird.
Particle inhalability curves in Menache et al., 1995, show that particulates as large as 100 µm may be able to enter the nares of the laboratory rat. Particles at the high end of this range would likely not be respired; however, they will enter the respiratory tract and be deposited on the epithelium at various locations lining the airway. In addition, HED assumes that particles as large as 100 µm are also inhalable to humans. Due to the lack of inhalability data for birds, these taxa are assumed to be able to inhale the same size of particle as mammalian taxa. Therefore, for both mammals and birds, STIR assumes 100 µm to be the upper particulate size limit of inhalability.
After entering the respiratory tract, there are a number of ways that particulates are cleared from the airway, including the absorption of the material across the epithelium lining the airway (U.S. EPA, 1996). Current HED methods to extrapolate from the mammalian oral route to the inhalation route incorporate the assumption of 100% absorption. At the screening level of STIR, the assumption of 100% absorption therefore provides a conservative estimate of potential pesticide spray inhalation exposure.
Several assumptions were made to define the duration of exposure for both droplet and vapor inhalation 2. The model operates under the assumption that spray droplets are homogenously scattered throughout the air column during spray events up to a specified height based on the method of application. Aerial sprays are assumed to be from a height of 3.3 m, which is the Tier I default release height above a canopy from the AgDrift model. Ground sprays are assumed to be released from 1 m in height, which is between the Tier I AgDrift boom heights of 0.5 m (low boom) and 1.2 m (high boom). The droplet inhalation exposure is assumed to be 1.5 minutes for aerial applications and 30 seconds for ground sprays, whereas the vapor inhalation exposure duration is assumed to be one hour. AgDrift was used to derive the assumptions of droplet inhalation duration by identifying the size of the default agricultural field based on an aerial application of a pesticide over 20 swaths with each swath being 18.3 m in width. Given a 10 mph wind based on default AgDrift assumptions, a spray droplet of median size in the "very fine" category (approximately 81.5 µm) can remain suspended in air nearly the entire distance of the field in just under 1.5 minutes. Ground applications with the same assumptions showed that a spray droplet of median size can stay suspended in air for just under 30 seconds. However, this screening tool did not incorporate variations in specific application and meteorological parameters such as water versus oil based carriers, humidity, wind speed, plant height, temperature, and particle size distributions. Each of these factors will influence the time that spray droplets will remain suspended in air.
STIR assumes one hour of vapor inhalation exposure and that the vapor phase is also homogenously distributed throughout the air column relevant to the location of a bird or mammal near the ground. One hour is a conservative estimate that assumes the maximum saturated air concentration (theoretical pure product air concentration) will persist for this time period. The level of exposure of the pesticide cannot be greater than the assumed saturated air concentration. STIR may therefore overestimate the actual air concentration found in the field. However, it provides a reasonable conservative estimate of vapor phase exposure duration at the screening level.
The model has a few other limitations related to exposure, extrapolation to other species, and chronic risk. STIR does not assess risk from particulate-bound pesticides (dust). Even though some birds take "dust baths" where they can agitate the soil into the air, there are no tools that have been evaluated by EFED that address this route of exposure at this time. In addition, the model only addresses acute inhalation risk to avian and mammalian species. Avian species typically serve as a surrogate to reptiles and terrestrial-phase amphibians. However, there is a significant difference in the respiratory biology of birds and amphibians/reptiles (Maina, 2003), which would require more research to assess the ability to extrapolate from avian to amphibian/reptile inhalation toxicity. Therefore, risk to other taxa, e.g. reptiles, terrestrial-phase amphibians, and plants remain an uncertainty. Finally, the model also does not address chronic toxicity. Birds and mammals may be exposed over longer periods of time to pesticides that volatilize or remain as suspended droplets, as well as pesticides with multiple applications. Chronic and bioaccumulation risks to avian and mammalian species through inhalation exposure therefore also remain an uncertainty.
2AgDrift was used to obtain information about the length of time that droplet particles of different sizes would remain aloft. The "Drop Distance" tool under AgDrift Tier II analysis was used with the default assumptions of a 10 mph wind, 85° F temperature, 50% relative humidity, and release heights of 3.3m and 1m depending on the type of application. The very fine droplet spectrum was used to determine the median particle size, which was incorporated into the Time Drop tool. The default assumption of 20 swaths of 60 ft width was used to determine the size of the field. This information was used to determine the length of time a droplet particle of a specified diameter could remain suspended aloft on the field before either being deposited or transported off-site.
References
Driver, C.J., M.W. Ligotke, P. Van Voris, B.D. McVeety, and D.B. Brown. 1991. Routes of uptake and their relative contribution to the toxicologic response of northern bobwhite (Colinus virginianus) to an organophosphate pesticide. Environmental Toxicology and Chemistry 10:21-33.
Lasiewski, R.C. and W.A. Calder. 1971. A preliminary allometric analysis of respiratory variables in resting birds. Respiration Physiology. 11:152-166.
Lasiewski, R.C. and W.R. Dawson. 1967. A reexamination of the relation between standard metabolic rate and body weight in birds. Condor. 69:12-23.
Maina, J.N. 2005. The Lung-Air Sac System of Birds: Development, Structure, and Function. Springer: New York. 210 pp.
Menache, M.G., F.J. Miller, and O.G. Raabe. 1995. Particle Inhalability Curves for Humans and Small Laboratory Animals. Ann. Occup. Hyg. 39(3): 317-328.
Mineau, P., B.T. Collins, and A. Baril. 1996. On the use of scaling factors to improve interspecies extrapolation to acute toxicity in birds. Reg. Toxicol. Pharmacol. 24: 24-29.
Nagy, K.A. 1987. Field metabolic rate and food requirement scaling in mammals and birds. Ecological Monograph. 57: 111-128.
Pepelko, W. and J.R. Withey. 1985. Methods for Route-to-Route Extrapolation of Dose. In: Toxicology and Industrial Health. Volume 1 (4). Pages 153-170.
Sample, B.E. and C.A. Arenal. 1999. Allometric Models for Interspecies Extrapolation of Wildlife Toxicity Data. Bulletin of Environmental Contamination and Toxicology. 62:653-663.
USEPA. 1993. Wildlife Exposure Factors Handbook. United States Environmental Protection Agency. Office of Research and Development. EPA/600/R-93/187.
USEPA. 1996. Air Quality Criteria for Particulate Matter (Final Report, April 1996). U.S. Environmental Protection Agency, Washington, D.C., EPA 600/P-95/001.
USEPA. 2004a. Scientific Advisory Panel. March 30 - April 2, 2004: Refined (Level II) Terrestrial and Aquatic Models for Probabilistic and Ecological Assessment of Pesticides. Available: FIFRA Scientific Advisory Panel Historical Meetings.
USEPA. 2004b. Procedure for the Inclusion of Open Literature Searches in Pesticide Screening Level Risk Assessments for Ecological Effects. U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, Washington DC. January 21, 2004.
USEPA. 2004c. Interim Guidance of the Evaluation Criteria for Ecological Toxicity Data in the Open Literature. Support Document J.2. U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, Washington DC. July, 2004.
USEPA. 2007. ECOTOX User Guide: ECOTOXicology Database System. Version 4.0. Available: http://cfpub.epa.gov/ecotox/
