Guidance for Using Non-Definitive Endpoints in Evaluating Risks to Listed and Non-listed Animal Species
On this page
- Memorandum
- Guidance for Using Non-Definitive Endpoints in Evaluating Risks to Listed and Non-listed Animal Species
- APPROACH FOR ADDRESSING NON-DEFINITIVE TOXICITY ENDPOINTS FOR ANIMALS IN PROBLEM FORMULATION
-
-
No Definitive LC50/EC50/LD50 Available for the Taxon; Non-definitive LC50/EC50/LD50 ≤ EECs
-
- Mortality noted
- Sublethal effects (no mortality) noted and the highest concentration tested is less than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s)
- Sublethal effects (no mortality) noted and the highest concentration tested is greater than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentrations(s)
-
- Chronic Endpoints
-
-
APPROACH FOR ADDRESSING NON-DEFINITIVE TOXICITY ENDPOINTS IN RISK ASSESSMENT
-
-
Non-definitive LC50/EC50/LD50 > EECs (No Mortality or Sublethal Effects)
-
Non-definitive LC50/EC50/LD50 > EECs (Mortality and/or Sublethal Effects Noted)
- Sublethal effects (no mortality) noted and the highest concentration tested is less than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s)
- Sublethal effects (no mortality) noted and the highest concentration tested is greater than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s)
- Chronic Endpoints
-
- APPROACH FOR ADDRESSING NON-DEFINITIVE TOXICITY ENDPOINTS FOR ANIMALS IN PROBLEM FORMULATION
MEMORANDUM
May 10, 2011
SUBJECT: Guidance for Using Non-Definitive Endpoints in Evaluating Risks to Listed and Non-listed Animal Species
FROM: /s/ Donald J. Brady, Ph.D., Director, Environmental Fate and Effects Division
TO: All Managers and Staff of the Environmental Fate and Effects Division
Please find the attached document entitled Guidance for Using Non-Definitive Endpoints in Evaluating Risks to Listed and Non-Listed Animal Species. This guidance is effective immediately.
This guidance is intended to ensure consistency among OPP scientists in the use of non-definitive endpoints (i.e., toxicity endpoints that have "less than" or "greater than" values) when conducting ecological risk assessments for pesticides and federally listed and/or non-listed species. This guidance was specifically developed to be used during Registration Review, although it can be used to determine the need for additional data and/or data gaps as part of the risk assessment process for new chemicals, new uses, litigation, FIFRA Section 18 emergency exemptions and Section 24(c) Special Local Need registrations. This guidance is specifically for addressing non-definitive toxicity endpoints for terrestrial and aquatic animals.
This guidance was discussed at three Chemical Review Process (CRP) meetings (on March 18, March 30, and June 8, 2010). As a result of those meetings the decision was made to complete development and issuance of this guidance specific to animal end-point and to address plant end-points separately. Further, it was pointed out that this guidance will require staff to calculate estimated environmental concentrations (EECs) at the Problem Formulation phase of the Registration Review process. I realize this will require a change in approach but believe it is a necessary change and will result in a more efficient process overall.
After the June 8 CRP meeting, one major issue remained unresolved - When to recommend calling in data for non-definitive acute and sub-acute endpoints that are the result of limit tests.
On Aug. 2, 2010, EFED staff provided an information briefing for OPP Division Directors (HED, PRD, and RD were represented) on the draft non-definitive endpoint guidance in which 2 options were presented to address this issue. These options were to call in additional data when:
-
the available non-definitive endpoint is from a limit test with fewer than 30 tested individuals (due to the statistical uncertainty associated with the small n), regardless of whether or not there were treatment-related effects noted in the study; and the highest concentration tested was not ≥ 10X (for mammals and birds) or 20X (for fish and invertebrates) the EEC.
OR
-
the available non-definitive endpoint is from a limit test with fewer than 30 tested individuals and there are frank sublethal effects and/or mortality noted in the limit test and the highest concentration tested was not ≥ 10X (for mammals and birds) or 20X (for fish and invertebrates) the EEC.
Since that time, a third approach has been identified - calling in additional data when the highest concentration tested is ≤ the EEC; when the highest concentration tested is ≥ the EEC and there is any treatment-related mortality noted in the limit test; or when the highest concentration tested is ≥ the EEC but not ≥ 10X or 20X the EEC and there are frank sublethal effects noted in the study.
I believe this third approach best fits with OPP risk assessment needs, is more closely aligned with our current testing guidelines, and is the most scientifically robust. For these reasons, this third option is the approach adopted in the attached non-definitive endpoint guidance.
The effort to develop non-definitive endpoint guidance was led by the Endangered Species Registration Review Workgroup with input and assistance from, members of EFED's Terrestrial Biology Technical Team and the Aquatic Biology Technical Team, and EFED management and senior staff. Any questions should be directed to Melissa Panger.
Attachment
Guidance for Using Non-Definitive Endpoints in Evaluating Risks to Listed and Non-listed Animal Species
May 6, 2011
Environmental Fate and Effects Division
Office of Pesticide Programs
U.S. Environmental Protection Agency
The Agency's pesticide ecological testing guidelines allow for "limit tests" for acute and sub-acute exposures (e.g., testing a chemical up to 2,000 mg a.i./kg-bw for birds and mammals, 100 mg a.i./L for aquatic organisms, and 25 µg a.i./bee for honey bees). Because only one concentration is typically tested in a limit test, a definitive LC50/EC50/LD50 value cannot be calculated from these studies. Additionally, some acute and/or sub-acute studies fail to demonstrate a definitive endpoint because an LC50/EC50/LD50 value cannot be calculated based on the effects observed at the concentrations tested. If mortality does not reach 50% at the highest concentration tested, the resulting LC50/EC50/LD50 is a "greater than" value (e.g., LD50 >2,000 mg a.i./kg-bw), and the concentration that would result in 50% mortality is unknown. In some cases, relevant estimated environmental concentrations (EECs) for a pesticide with a non-definitive acute and/or sub-acute toxicity endpoint are higher than the highest concentration tested.
For chronic studies, some chronic animal studies fail to identify a NOAEC because growth, reproductive, and/or mortality effects are observed at the lowest concentration tested. The resulting NOAEC is a "less than" value (e.g., NOAEC < 50 mg a.i./kg-bw), and the concentration that would result in no effects is unknown. Guidance for addressing non-definitive toxicity endpoints for animals is needed for consistency among EFED scientists. This guidance was specifically developed to be used during Registration Review, although it can be used to determine the need for additional data and/or data gaps as part of the risk assessment process for new chemicals, new uses, litigation, Section 18s and Section 24cs. This guidance is specifically for addressing non-definitive toxicity endpoints for terrestrial and aquatic animals; guidance for addressing non-definitive endpoints for terrestrial and aquatic plants will be developed in a separate document.
-
APPROACH FOR ADDRESSING NON-DEFINITIVE TOXICITY ENDPOINTS FOR ANIMALS IN PROBLEM FORMULATION
Acute and Sub-Acute Endpoints
Guidance for addressing non-definitive acute and sub-acute endpoints in Problem Formulation (PF) stage of Registration Review, based on the "decision tree" illustrated below (Figure 1), is provided in the following sections.
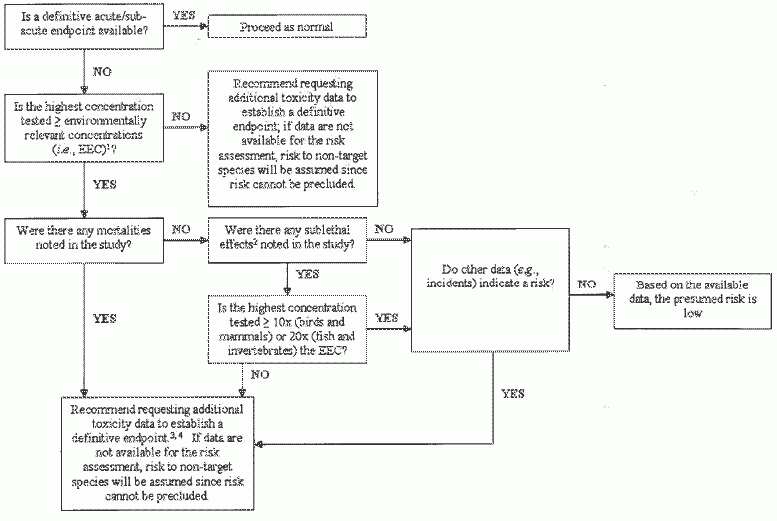 Figure 1
Figure 1
Decision Tree to Address Non-definitive Acute and Sub-acute Toxicity Endpoints in PF1 If the EEC in aquatic habitats exceeds a chemical's water solubility limit, then the environmentally relevant concentration should be considered the assumed water solubility limit (i.e., the EEC for aquatic environments should not exceed the solubility limit).
2 "'Sublethal" effects here refer to frank toxicological effects (e.g., decreased body weight, loss of coordination, erratic swimming, lethargy) and not to less significant sublethal effects (e.g., ruffled appearance, muted color).
3 For aquatic species, the maximum test concentration should be the solubility limit in situations where the solubility limit is less than 20x the EEC. For terrestrial species, the maximum test concentration should be based on the limit that is physiologically practical in situations where 10x the EEC level exceeds the digestive capacity of the test organisms.
4 Data call-in is recommended; however, any registrant-submitted waiver that uses alternative lines of toxicological evidence (e.g., QSAR or chemical analogue analysis) to provide a defensible definitive endpoint will be considered by the Agency.
Special note for chemicals with solubility limits < 100 mg/L
For chemicals with water solubility limits < 100 mg/L, the water solubility limit should be considered when addressing non-definitive aquatic organism toxicity endpoints for acute exposures. In the guidance provided below, if studies have been conducted with concentrations greater than the water solubility limit of the chemical, then the highest concentration tested should be considered the assumed water solubility limit (i.e., the highest concentration tested should not exceed the solubility limit). Additionally, if calculations of environmentally relevant concentrations1 in aquatic habitats exceed a chemical's water solubility limit, then the environmentally relevant concentration should be considered the assumed water solubility limit (i.e., the environmentally relevant concentration for aquatic environments should not exceed the solubility limit). An exception would be if data are available that demonstrate that exposure concentrations may exceed the laboratory water solubility concentration. In these cases, it may not be reasonable to cap the concentrations at the laboratory water solubility limit.
Special note for calculating environmentally relevant concentrations in the Problem Formulation:
When comparing tested concentrations from studies with non-definitive endpoints to environmentally relevant concentrations [i.e., estimated environmental concentrations (EEC)] in the Problem Formulation, calculate EECs for the use that would result in the highest EECs for your chemical. Typically this would be the use with the highest application rates and/or shortest application intervals. For calculating aquatic EECs (non-rice and non-aquatic uses), use GENEEC2. For applications to rice, use the Rice Model to calculate EECs. For applications applied directly to water use the target concentration specified on the label as the EEC. For estimating terrestrial EECs, run T-REX for birds and mammals using a small (20g) bird and (15g) mammal that eat short grass, respectively. Use the T-REX "small insect" EECs to estimate exposures to terrestrial invertebrates.
1 Environmentally relevant concentrations are those that are expected to occur in the environment with the use of the pesticide (i.e., estimated environmental concentrations, EEC).
2 EECs calculated using PRZM/EXAMS or EECs from a recent assessment that reflect conservative EECs for the range of uses in the problem formulation may also be used.
-
No Definitive LC50/EC50/LD50 Available for the Taxon; Non-definitive LC50/EC50/LD50 ≤ EECs
If there are results from more than one study available for a particular taxon (and no definitive endpoint is available), use the highest dose tested (or the chemical's solubility limit, if applicable). Compare this non-definitive endpoint to environmentally relevant concentrations (e.g., EECs).
If the highest concentration tested is less than the environmentally relevant concentration(s), risks to the taxon of concern cannot be precluded. Therefore, until a definitive endpoint for acute exposure is available, risks to the taxon of concern from the use of Pesticide X will be assumed. A request for new data to establish a definitive endpoint should be recommended.
An example of text that describes the type of information to include in the PF regarding the taxon of concern when the highest concentration tested is less than the environmentally relevant concentration is provided below in Section I.A.1.
-
The highest concentration tested is less than the environmentally relevant concentration
A definitive LC50 [or LD50 or EC50] has not been established for [taxon of concern]. In order to gain a better understanding of how the EECs for the maximum proposed Pesticide X application rate relate to the toxicity data currently available for [taxon], the [insert appropriate exposure model] is used to calculate a ratio of the EEC and toxicity endpoint using the conservative assumption that the highest value tested represents the toxicity endpoint. In this exercise, all [or some] of the acute [and sub-acute, as applicable] ratios calculated are > 1. Because the highest dose tested is below the highest predicted exposure level, the specific risk to [taxon] from the use of Pesticide X is unknown. Therefore, in order to complete the risk assessment, additional data are needed. In the absence of such data, risks to both listed and non-listed [taxon] species will be assumed in the risk assessment conducted for Registration Review.
-
-
No Definitive LC50/EC50/LD50 Available for the Taxon; Non-definitive LC50/EC50/LD50 > EECs (No Mortality or Sublethal Effects)
If there are results from more than one study available for a particular taxon (and no definitive endpoint is available), use the highest dose tested (or the chemical's solubility limit, if applicable to determine if the endpoint would be higher than the exposure estimates (if there were mortalities or sublethal effects noted at the highest concentration tested, see Section I.C). Compare this non-definitive endpoint to environmentally relevant concentrations (e.g., EECs).
If the highest concentration tested (with no mortality or frank sublethal effects) is greater than the environmentally relevant concentrations, assume that the risk from acute exposure is low based solely on the available toxicity data and estimated exposures. Assessors should consider all available information (e.g., incident data, monitoring data) to make their risk call.
Assessors should be aware that with limit tests the statistical uncertainty associated with the results increases as the number of individuals included in the study decreases. For example, based on the Binomial Theorem (95% confidence limits), in a limit test with 7 treated individuals, there is a 5% chance that the true proportion of mortality at the limit dose is actually 41%, even if no effects were noted in the study. Therefore, with limit tests that include at least 7 treated individuals, there is statistical confidence that the actual LC50/LD50/EC50 value is > the limit dose. For limit tests with fewer than 7 treated individuals, even if no effects are noted in the study, there is a chance (which increases as the number of individuals decreases) that the true proportion of mortality at the limit dose is > 50%.
Examples of text that describe the type of information to include in the PF regarding the taxon of concern when all of the available data indicate a low potential for risk and when some of the available data indicate the potential for risk are provided below in Sections I.B.1 and I.B.2, respectively.
-
All available data indicate low potential for risk
Because no mortality or sublethal effects were observed at the highest treatment levels tested, standard RQ values for acute [and sub-acute, if applicable] exposure(s) are not calculated. In order to gain a better understanding of how the EECs for the maximum proposed Pesticide X application rate relate to the toxicity data currently available for [taxon], the [insert appropriate exposure model] is used to calculate a ratio of the EEC and toxicity endpoint using the conservative assumption that the highest value tested represents the toxicity endpoint. In this exercise, all of the acute [and sub-acute, as applicable] ratios calculated are < 1. Actual RQs would likely be much lower because no effects were observed at the highest treatment levels tested. [Discuss other available information - e.g., incident data, monitoring data, and/or relevant open literature data]. Therefore, based on the available information, risk to [taxon] from acute exposure to Pesticide X is not expected. A request for new data to establish a definitive endpoint is not recommended at this time.
-
Some available data indicate a potential for risk
Because no mortality or sublethal effects were observed at the highest treatment levels tested, standard RQ values for acute [and sub-acute, if applicable] exposure(s) are not calculated. In order to gain a better understanding of how the EECs for the maximum proposed Pesticide X application rate relate to the toxicity data currently available for [taxon], the [insert appropriate exposure model] is used to calculate a ratio of the EEC and toxicity endpoint using the conservative assumption that the highest value tested represents the toxicity endpoint. In this exercise, all of the acute [and sub-acute, as applicable] ratios calculated are < 1 [indicating that the EECs are less than the highest concentration tested (at which there were no toxic effects noted)]. Actual RQs would likely be much lower because no effects were observed at the highest treatment levels tested. However, [Discuss other available information - e.g., incident data, monitoring data, and/or relevant open literature data] based on the available information, the potential risk to [taxon] from acute exposure to Pesticide X exists. Therefore, until a definitive endpoint for acute exposure is available, risks to [taxon] from the use of Pesticide X will be assumed [recommend DCI - see below].
-
-
No Definitive LC50/EC50/LD50 Available for the Taxon; Non-definitive LC50/EC50/LD50 > EECs (Mortality and/or Sublethal Effects Noted)
If there are results from more than one study available for a particular taxon (and no definitive endpoint is available), use the highest dose tested (or the chemical's solubility limit, if applicable). Compare this non-definitive endpoint to environmentally relevant concentrations (e.g., EECs).
If the highest concentration tested is greater than the environmentally relevant concentration(s) and there were treatment-related mortalities noted in the study, risks to the taxon of concern cannot be precluded. Therefore, until a definitive endpoint for acute exposure is available, risks to the taxon of concern from the use of Pesticide X will be assumed. A request for new data to establish a definitive endpoint should be recommended (see I.C.1. below).
If the highest concentration tested is greater than the environmentally relevant concentration(s) and there were treatment-related frank sublethal effects (e.g., decreased body weight, loss of coordination, erratic swimming) noted in the study, the assessor will need to determine if the highest concentration tested is less than or greater than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s) (see I.C.2. and I.C.3. below). These factors are based on the current listed species LOCs for birds and mammals (i.e., RQ > 0.1 or 1/10th the acute/sub-acute endpoint value) and fish and invertebrates (i.e., RQ > 0.05 or 1/20th of the acute endpoint value).
-
Mortality noted
If the highest concentration tested is greater than the environmentally relevant concentration(s) and there was treatment-related mortality noted in the study, risks to the taxon of concern cannot be precluded. Therefore, until a definitive endpoint for acute exposure is available, risks to the taxon of concern from the use of Pesticide X will be assumed. A request for new data to establish a definitive endpoint should be recommended.
Example text that describes the type of information to include in the PF regarding the taxon of concern when the highest concentration tested is greater than the environmentally relevant concentration(s) and there was treatment-related mortality noted in the study is provided below.
Although a definitive LC50[or LD50 or EC50] was not established in the study, mortality [and sublethal effects (list effects) if any] were noted. In order to gain a better understanding of how the EECs for the maximum proposed Pesticide X application rate relate to the toxicity data currently available for [taxon], the [insert appropriate exposure model] is used to calculate a ratio of the EEC and toxicity endpoint using the conservative assumption that the highest value tested represents the toxicity endpoint. In this exercise, all of the acute [and sub-acute, as applicable] ratios calculated are > [0.1 (for birds and mammals) or 0.05 (for fish and invertebrates)]. Although the highest dose tested is above the maximum expected exposure level, the study indicates that there is a risk of mortality to non-target [taxon] from the use of this pesticide. Therefore, the risk to federally listed species from the use of Pesticide X cannot be precluded. Therefore, in order to complete the risk assessment, additional data are needed. In the absence of such data, risks to listed species will be assumed.
-
Sublethal effects (no mortality) noted and the highest concentration tested is less than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s)
If the highest concentration tested is less than 10x (for birds and mammals) or 20x (for fish and invertebrates) environmentally relevant concentration(s), and there were treatment-related frank sublethal effects (e.g., decreased body weight, loss of coordination, erratic swimming), risks to federally listed species cannot be precluded. Therefore, until a definitive endpoint for acute exposure is available, risks to listed species (belonging to the taxon of concern) from the use of Pesticide X will be assumed. A request for new data to establish a definitive endpoint should be recommended. For aquatic species, the maximum test concentration should be the solubility limit in situations where the solubility limit is less than 20x the EEC. For terrestrial species, the maximum test concentration should be based on the limit that is physiologically practical in situations where 10x the EEC level exceeds the digestive capacity of the test organisms. Data call-in is recommended; however, any registrant-submitted waiver that uses alternative lines of toxicological evidence (e.g., QSAR or chemical analogue analysis) to provide a defensible definitive endpoint will be considered by the Agency.
Example text that describes the type of information to include in the PF regarding the taxon of concern when the highest concentration tested is less than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s), and there were treatment-related mortalities and/or sublethal effects noted in the study is provided below.
Although a definitive LC50[or LD50 or EC 50] was not established in the study, the following effects were seen: [list effects]. In order to gain a better understanding of how the EECs for the maximum proposed Pesticide X application rate relate to the toxicity data currently available for [taxon], the [insert appropriate exposure model] is used to calculate a ratio of the EEC and toxicity endpoint using the conservative assumption that the highest value tested represents the toxicity endpoint. In this exercise, all [or some] of the acute [and sub-acute, as applicable] ratios calculated are > [0.1 (for birds and mammals) or 0.05 (for fish and invertebrates)]. Because the highest dose tested is below the exposure level required to preclude risk to federally listed species, the specific risk to listed [taxon] from the use of Pesticide X is unknown. Therefore, in order to complete the risk assessment, additional data are needed. In the absence of such data, risks to listed species will be assumed.
-
Sublethal effects (no mortality) noted and the highest concentration tested is greater than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentrations(s)
If the highest concentration tested is greater than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s), risk to federally listed species is assumed to be low based solely on the available toxicity data and estimated exposures. Assessors should consider all available information (e.g., incident data, monitoring data) to make their risk call.
Example text is provided below that describes the type of information to include in the PF regarding the taxon of concern when all of the available data indicate a low potential for risk to listed species (I.C.3.a) and when some of the available data indicate the potential for risk to listed species (I.C.3.b).
-
All available data indicate low potential for risk to listed species
In order to gain a better understanding of how the EECs for the maximum proposed Pesticide X application rate relate to the toxicity data currently available for [taxon], the [insert appropriate exposure model] is used to calculate a ratio of the EEC and toxicity endpoint using the conservative assumption that the highest value tested represents the toxicity endpoint. In this exercise, all [or some] of the acute [and sub-acute, as applicable] ratios calculated are < [0.1 (for birds and mammals) or 0.05 (for fish and invertebrates)]. [Discuss other available information - e.g., incident data, monitoring data, and/or relevant open literature data]. Therefore, based on the available information, risk to listed [taxon] from acute exposure to Pesticide X is not expected. A request for new data to establish a definitive endpoint is not recommended at this time.
-
Some available data indicate a potential for risk to listed species
In order to gain a better understanding of how the EECs for the maximum proposed Pesticide X application rate relate to the toxicity data currently available for [taxon], the [insert appropriate exposure model] is used to calculate a ratio of the EEC and toxicity endpoint using the conservative assumption that the highest value tested represents the toxicity endpoint. In this exercise, all of the acute [and sub-acute, as applicable] ratios calculated are < [0.1 (for birds and mammals) or 0.05 (for fish and invertebrates)].
However, [Discuss other available information - e.g., incident data, monitoring data, and/or relevant open literature data]. Based on the available information, the potential risk to listed [taxon] from acute exposure to Pesticide X cannot be precluded. Therefore, until a definitive endpoint for acute exposure is available, risks to listed [taxon] from the use of Pesticide X will be assumed [recommend DCI - see below].
-
-
Chronic Endpoints
Some chronic animal studies fail to identify a NOAEC because growth, reproductive, and/or mortality effects are observed at the lowest concentration tested. The resulting NOAEC is a "less than" value (e.g., NOAEC < 50 mg a.i./kg-bw), and the concentration that would result in no effects is unknown. Therefore, risks to federally listed species cannot be precluded. Until a definitive endpoint for chronic exposure is available, risks to listed and non-listed species (belonging to the taxon of concern) from the use of Pesticide X will be assumed. A request for new data to establish a definitive endpoint should be recommended.
An example of text that describes the type of information to include in the PF regarding the taxon of concern when a definitive NOAEC is not available because effects were seen at the lowest concentration tested is provided below.
A definitive NOAEC has not been established for [taxon of concern]. Because the lowest dose tested resulted in effects, the specific risk to [taxon] from chronic exposure to Pesticide X is unknown. Therefore, in order to complete the risk assessment, additional data are needed. In the absence of such data, risks to both listed and non-listed [taxon] species will be assumed in the risk assessment conducted for Registration Review.
-
-
APPROACH FOR ADDRESSING NON-DEFINITIVE TOXICITY ENDPOINTS IN RISK ASSESSMENT
Acute and Sub-Acute Endpoints
If, at the time of conducting the risk assessment, only non-definitive acute and/or sub-acute endpoints are available for a particular taxon, do not calculate acute (or sub-acute) RQs for the taxon in the Risk Estimation section of the assessment. The non-definitive endpoints should be discussed and compared to environmentally relevant concentrations in the Risk Description section of the assessment. Follow the recommendations outlined below:
-
Non-definitive LC50/EC50/LD50 ≤ EECs
If the highest concentration tested is less than the environmentally relevant concentration(s), risks to the taxon of concern cannot be precluded. Therefore, assume risks to the taxon of concern (both listed and non-listed species) from the use of Pesticide X.
-
Non-definitive LC50/EC50/LD50 > EECs (No Mortality or Sublethal Effects)
If the highest concentration tested [with no mortality or frank sublethal effects (e.g., decreased body weight, loss of coordination, erratic swimming)] is greater than the environmentally relevant concentrations, assume that the risk from acute exposure is low (for both listed and non-listed species) based solely on the available toxicity data and estimated exposures. Assessors should consider all available information (e.g., incident data, monitoring data) to make their risk call.
-
Non-definitive LC50/EC50/LD50 > EECs (Mortality and/or Sublethal Effects Noted)
If the highest concentration tested is greater than the environmentally relevant concentration(s) and there was treatment-related mortality noted in the study, risks to the taxon of concern cannot be precluded. Therefore, until a definitive endpoint for acute exposure is available, risks to the taxon of concern from the use of Pesticide X will be assumed.
If the highest concentration tested is greater than the environmentally relevant concentration(s) and there were treatment-related frank sublethal effects (e.g., decreased body weight, loss of coordination, erratic swimming) noted in the study, the assessor will need to determine if the highest concentration tested is less than or greater than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s) (see II.C.a. and II.C.b. below).
-
Sublethal effects (no mortality) noted and the highest concentration tested is less than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s)
If the highest concentration tested is less than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s), and there were treatment-related sublethal effects in the study, risks to federally listed species cannot be precluded and should be assumed.
-
Sublethal effects (no mortality) noted and the highest concentration tested is greater than 10x (for birds and mammals) or 20x (for fish and invertebrates) the environmentally relevant concentration(s)
If the highest concentration tested is greater than 10x (for birds and mammals) or 20x (for fish and invertebrates) environmentally relevant concentration(s) and there was no mortality in the study, risks to federally listed species is assumed to be low based solely on the available toxicity data and estimated exposures. Assessors should consider all available information (e.g., incident data, monitoring data) to make their risk call.
-
Chronic Endpoints
If, at the time of conducting the risk assessment, only a non-definitive chronic endpoint(s) is available for a particular taxon, do not calculate RQs for the taxon in the Risk Estimation section of the assessment. The non-definitive endpoint(s) should be discussed and compared to environmentally relevant concentrations in the Risk Description section of the assessment.
If the lowest concentration tested resulted in effects on growth, reproduction, and/or mortality, risks to the taxon of concern from chronic exposure cannot be precluded. Therefore, assume risks to the taxon of concern (both listed and non-listed species) from the use of Pesticide X.
Any questions should be directed to Melissa Panger or other members of the Workgroup.
-
Endangered Species Reregistration Workgroup
David Bays, AD
Shannon Borges, BPPD
James Breithaupt, AD
Mark Corbin, EFED
Kevin Costello, PRD
William Eckel, EFED
Catherine Eiden, PRD
Melissa Grable, EFED
Mark J. Huff, EFED
Stephanie Irene, EFED
Russell Jones, BPPD
Stephen Morrill, BPPD
Edward Odenkirchen, EFED (Co-Chair)
Melissa Panger, EFED
Anita Pease, EFED
Mohammed Ruhman, EFED
Dana Spatz, EFED
Thomas Steeger, EFED
Ingrid Sunzenauer, EFED (Co-Chair)
Michelle Thawley, EFED
Zigfridas Vaituzis, BPPD
Katrina White, EFED
