Climate Change Indicators: Atmospheric Concentrations of Greenhouse Gases
This indicator describes how the levels of major greenhouse gases in the atmosphere have changed over time.
-
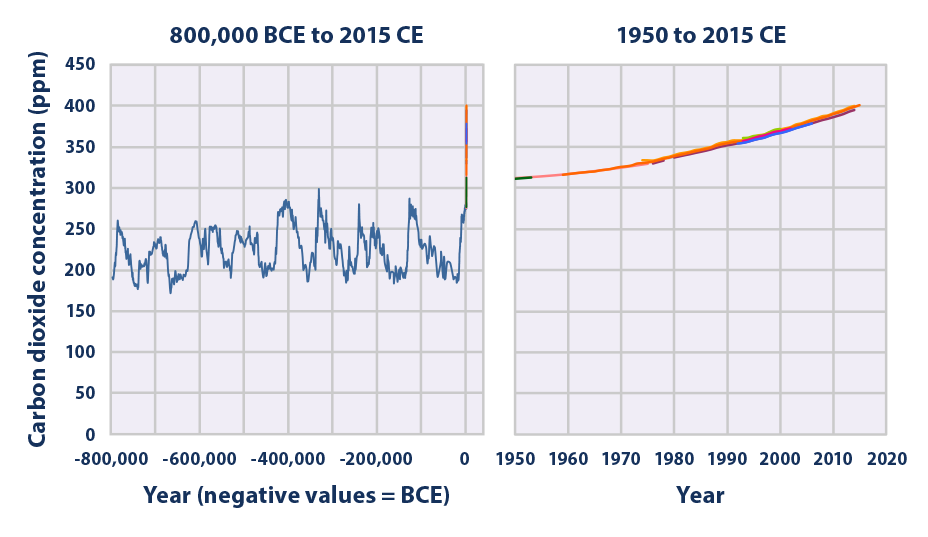
This figure shows concentrations of carbon dioxide in the atmosphere from hundreds of thousands of years ago through 2015, measured in parts per million (ppm). The data come from a variety of historical ice core studies and recent air monitoring sites around the world. Each line represents a different data source.
Data source: Compilation of 10 underlying datasets5
Web update: April 2016 -
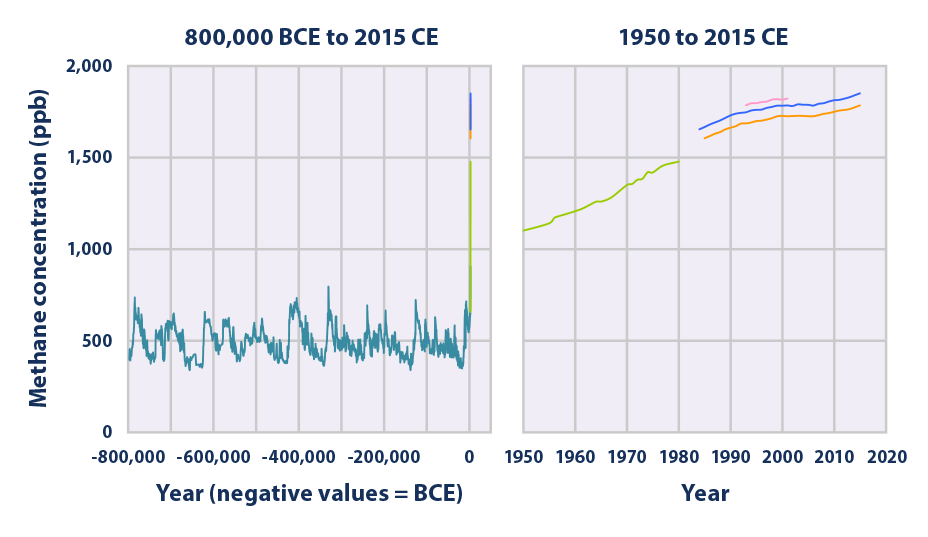
This figure shows concentrations of methane in the atmosphere from hundreds of thousands of years ago through 2015, measured in parts per billion (ppb). The data come from a variety of historical ice core studies and recent air monitoring sites around the world. Each line represents a different data source.
Data source: Compilation of five underlying datasets6
Web update: August 2016 -
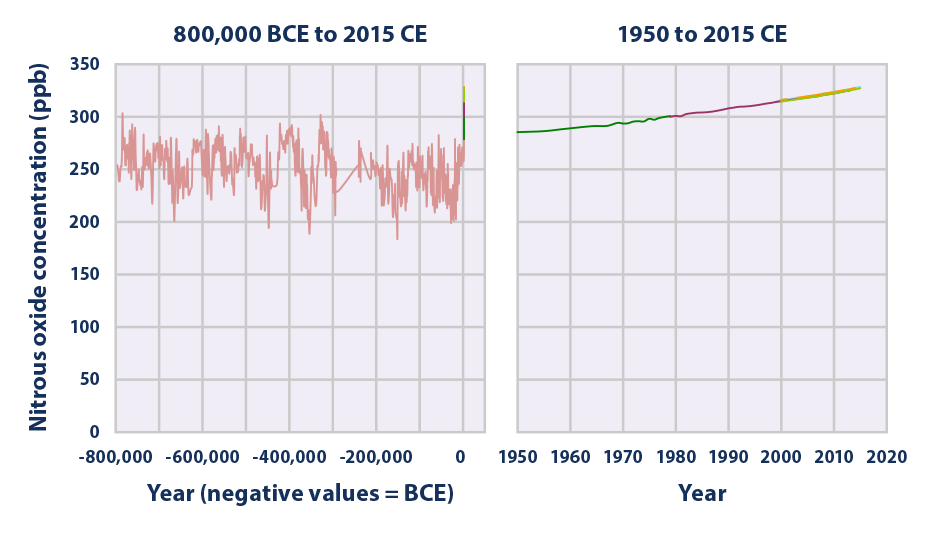
This figure shows concentrations of nitrous oxide in the atmosphere from hundreds of thousands of years ago through 2015, measured in parts per billion (ppb). The data come from a variety of historical ice core studies and recent air monitoring sites around the world. Each line represents a different data source.
Data source: Compilation of six underlying datasets7
Web update: August 2016 -
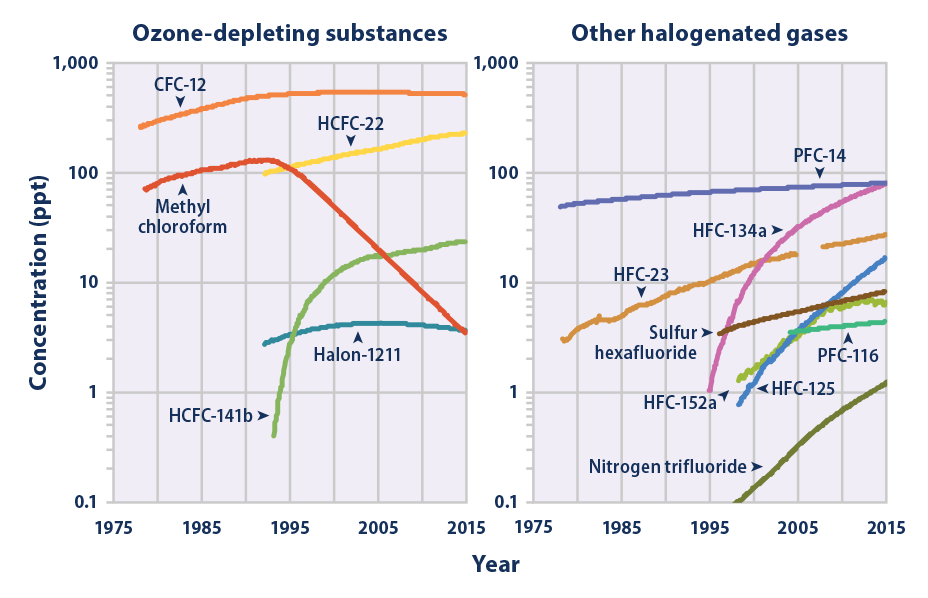
This figure shows concentrations of several halogenated gases (which contain fluorine, chlorine, or bromine) in the atmosphere, measured in parts per trillion (ppt). The data come from monitoring sites around the world. Note that the scale increases by factors of 10. This is because the concentrations of different halogenated gases can vary by a few orders of magnitude. The numbers following the name of each gas (e.g., HCFC-22) are used to denote specific types of those particular gases.
Data sources: AGAGE, 2016;8 Rigby, 2016;9 NOAA, 201610
Web update: August 2016 -
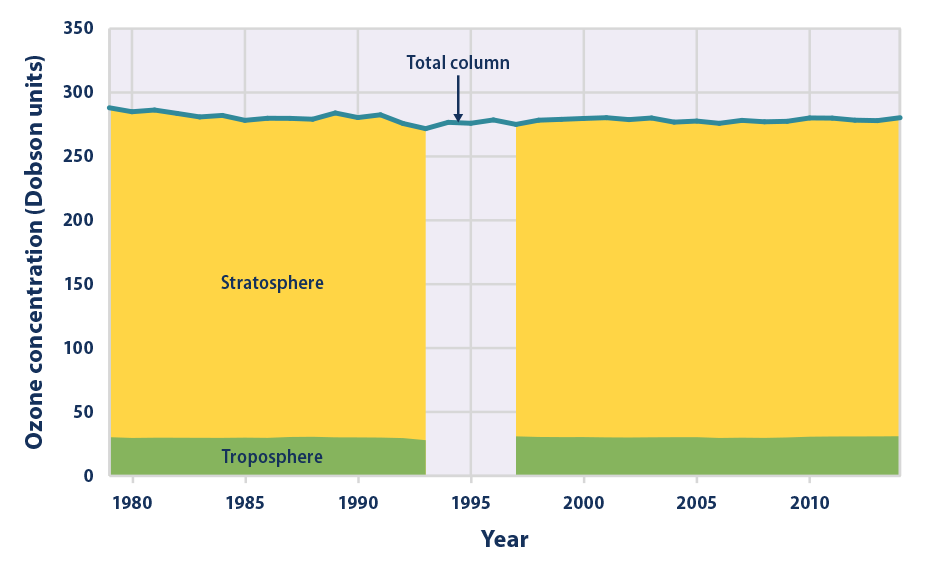
This figure shows the average amount of ozone in the Earth’s atmosphere each year, based on satellite measurements. The total represents the “thickness” or density of ozone throughout all layers of the Earth’s atmosphere, which is called total column ozone and measured in Dobson units. Higher numbers indicate more ozone. For most years, Figure 5 shows how this ozone is divided between the troposphere (the part of the atmosphere closest to the ground) and the stratosphere. From 1994 to 1996, only the total is available, due to limited satellite coverage.
Data sources: NASA, 2013,11 2015,12 201613
Web update: August 2016
Key Points
- Global atmospheric concentrations of carbon dioxide, methane, nitrous oxide, and certain manufactured greenhouse gases have all risen significantly over the last few hundred years (see Figures 1, 2, 3, and 4).
- Historical measurements show that the current global atmospheric concentrations of carbon dioxide, methane, and nitrous oxide are unprecedented compared with the past 800,000 years (see Figures 1, 2, and 3).
- Carbon dioxide concentrations have increased substantially since the beginning of the industrial era, rising from an annual average of 280 ppm in the late 1700s to 401 ppm as measured at Mauna Loa in 2015—a 43 percent increase (see Figure 1). Almost all of this increase is due to human activities.1
- The concentration of methane in the atmosphere has more than doubled since preindustrial times, reaching approximately 1,800 ppb in recent years (see the range of measurements for 2014 and 2015 in Figure 2). This increase is predominantly due to agriculture and fossil fuel use.2
- Over the past 800,000 years, concentrations of nitrous oxide in the atmosphere rarely exceeded 280 ppb. Levels have risen since the 1920s, however, reaching a new high of 328 ppb in 2015 (average of three sites in Figure 3). This increase is primarily due to agriculture.3
- Concentrations of many of the halogenated gases shown in Figure 4 were essentially zero a few decades ago but have increased rapidly as they have been incorporated into industrial products and processes. Some of these chemicals have been or are currently being phased out of use because they are ozone-depleting substances, meaning they also cause harm to the Earth’s protective ozone layer. As a result, concentrations of many major ozone-depleting gases have begun to stabilize or decline (see Figure 4, left panel). Concentrations of other halogenated gases have continued to rise, however, especially where the gases have emerged as substitutes for ozone-depleting chemicals (see Figure 4, right panel).
- Overall, the total amount of ozone in the atmosphere decreased by about 3 percent between 1979 and 2014 (see Figure 5). All of the decrease happened in the stratosphere, with most of the decrease occurring between 1979 and 1994. Changes in stratospheric ozone reflect the effect of ozone-depleting substances. These chemicals have been released into the air for many years, but recently, international efforts have reduced emissions and phased out their use.
- Globally, the amount of ozone in the troposphere increased by about 3 percent between 1979 and 2014 (see Figure 5).
Background
Since the Industrial Revolution began in the 1700s, people have added a substantial amount of greenhouse gases into the atmosphere by burning fossil fuels, cutting down forests, and conducting other activities (see the U.S. and Global Greenhouse Gas Emissions indicators). When greenhouse gases are emitted into the atmosphere, many remain there for long time periods ranging from a decade to many millennia. Over time, these gases are removed from the atmosphere by chemical reactions or by emissions sinks, such as the oceans and vegetation, which absorb greenhouse gases from the atmosphere. As a result of human activities, however, these gases are entering the atmosphere more quickly than they are being removed, and thus their concentrations are increasing.
Carbon dioxide, methane, nitrous oxide, and certain manufactured gases called halogenated gases (gases that contain chlorine, fluorine, or bromine) become well mixed throughout the global atmosphere because of their relatively long lifetimes and because of transport by winds. Concentrations of these greenhouse gases are measured in parts per million (ppm), parts per billion (ppb), or parts per trillion (ppt) by volume. In other words, a concentration of 1 ppb for a given gas means there is one molecule of that gas in every 1 billion molecules of air. Some halogenated gases are considered major greenhouse gases due to their very high global warming potentials and long atmospheric lifetimes even if they only exist at a few ppt (see table).
Ozone is also a greenhouse gas, but it differs from other greenhouse gases in several ways. The effects of ozone depend on its altitude, or where the gas is located vertically in the atmosphere. Most ozone naturally exists in the layer of the atmosphere called the stratosphere, which ranges from approximately 6 to 30 miles above the Earth’s surface. Ozone in the stratosphere has a slight net warming effect on the planet, but it is good for life on Earth because it absorbs harmful ultraviolet radiation from the sun, preventing it from reaching the Earth’s surface. In the troposphere—the layer of the atmosphere near ground level—ozone is an air pollutant that is harmful to breathe, a main ingredient of urban smog, and an important greenhouse gas that contributes to climate change (see the Climate Forcing indicator). Unlike the other major greenhouse gases, tropospheric ozone only lasts for days to weeks, so levels often vary by location and by season.
About the Indicator
This indicator describes concentrations of greenhouse gases in the atmosphere. It focuses on the major greenhouse gases that result from human activities.
For carbon dioxide, methane, nitrous oxide, and halogenated gases, recent measurements come from monitoring stations around the world, while measurements of older air come from air bubbles trapped in layers of ice from Antarctica and Greenland. By determining the age of the ice layers and the concentrations of gases trapped inside, scientists can learn what the atmosphere was like thousands of years ago.
This indicator also shows data from satellite instruments that measure ozone density in the troposphere, the stratosphere, and the “total column,” or all layers of the atmosphere. These satellite data are routinely compared with ground-based instruments to confirm their accuracy. Ozone data have been averaged worldwide for each year to smooth out the regional and seasonal variations.
Indicator Notes
This indicator includes several of the most important halogenated gases, but some others are not shown. Many other halogenated gases are also greenhouse gases, but Figure 4 is limited to a set of common examples that represent most of the major types of these gases. The indicator also does not address certain other pollutants that can affect climate by either reflecting or absorbing energy. For example, sulfate particles can reflect sunlight away from the Earth, while black carbon aerosols (soot) absorb energy. Data for nitrogen trifluoride (Figure 4) reflect modeled averages based on measurements made in the Northern Hemisphere and some locations in the Southern Hemisphere, to represent global average concentrations over time. The global averages for ozone only cover the area between 50°N and 50°S latitude (77 percent of the Earth’s surface), because at higher latitudes the lack of sunlight in winter creates data gaps and the angle of incoming sunlight during the rest of the year reduces the accuracy of the satellite measuring technique.
Data Sources
Global atmospheric concentration measurements for carbon dioxide (Figure 1), methane (Figure 2), and nitrous oxide (Figure 3) come from a variety of monitoring programs and studies published in peer-reviewed literature. Global atmospheric concentration data for selected halogenated gases (Figure 4) were compiled by the Advanced Global Atmospheric Gases Experiment, the National Oceanic and Atmospheric Administration, and a peer-reviewed study on nitrogen trifluoride. A similar figure with many of these gases appears in the Intergovernmental Panel on Climate Change’s Fifth Assessment Report.14 Satellite measurements of ozone were processed by the National Aeronautics and Space Administration and validated using ground-based measurements collected by the National Oceanic and Atmospheric Administration.
Technical Documentation
References
1. IPCC (Intergovernmental Panel on Climate Change). 2013. Climate change 2013: The physical science basis. Working Group I contribution to the IPCC Fifth Assessment Report. Cambridge, United Kingdom: Cambridge University Press. www.ipcc.ch/report/ar5/wg1.
2. IPCC (Intergovernmental Panel on Climate Change). 2013. Climate change 2013: The physical science basis. Working Group I contribution to the IPCC Fifth Assessment Report. Cambridge, United Kingdom: Cambridge University Press. www.ipcc.ch/report/ar5/wg1.
3. IPCC (Intergovernmental Panel on Climate Change). 2013. Climate change 2013: The physical science basis. Working Group I contribution to the IPCC Fifth Assessment Report. Cambridge, United Kingdom: Cambridge University Press. www.ipcc.ch/report/ar5/wg1.
4. IPCC (Intergovernmental Panel on Climate Change). 2013. Climate change 2013: The physical science basis. Working Group I contribution to the IPCC Fifth Assessment Report. Cambridge, United Kingdom: Cambridge University Press. www.ipcc.ch/report/ar5/wg1.
5. [see full list provided below]
6. [see full list provided below]
7. [see full list provided below]
8. AGAGE (Advanced Global Atmospheric Gases Experiment). 2016. ALE/GAGE/AGAGE data base. Accessed June 2016. http://agage.mit.edu/.
9. Rigby, M. 2016 update to data originally published in: Arnold, T., C.M. Harth, J. Mühle, A.J. Manning, P.K. Salameh, J. Kim, D.J. Ivy, L.P. Steele, V.V. Petrenko, J.P. Severinghaus, D. Baggenstos, and R.F. Weiss. 2013. Nitrogen trifluoride global emissions estimated from updated atmospheric measurements. P. Natl. Acad. Sci. USA 110(6):2029–2034. Data updated July 2016.
10. NOAA (National Oceanic and Atmospheric Administration). 2016. Halocarbons and Other Atmospheric Trace Species group (HATS). Accessed June 2016. www.esrl.noaa.gov/gmd/hats.
11. NASA (National Aeronautics and Space Administration). 2013. Data—TOMS/SBUV TOR data products. Accessed November 2013. http://science.larc.nasa.gov/TOR/data.html.
12. NASA (National Aeronautics and Space Administration). 2015. Tropospheric ozone data from AURA OMI/MLS. Accessed May 2015. http://acdb-ext.gsfc.nasa.gov/Data_services/cloud_slice/new_data.html.
13. NASA (National Aeronautics and Space Administration). 2016. SBUV merged ozone data set (MOD). Version 8.6. Accessed March 2016. http://acdb-ext.gsfc.nasa.gov/Data_services/merged/index.html.
14. IPCC (Intergovernmental Panel on Climate Change). 2013. Climate change 2013: The physical science basis. Working Group I contribution to the IPCC Fifth Assessment Report. Cambridge, United Kingdom: Cambridge University Press. www.ipcc.ch/report/ar5/wg1.
Atmospheric Concentrations of Greenhouse Gases: Citations for Figures 1, 2, and 3
Figure 1
EPICA Dome C and Vostok Station, Antarctica: approximately 796,562 BCE to 1813 CE
Lüthi, D., M. Le Floch, B. Bereiter, T. Blunier, J.-M. Barnola, U. Siegenthaler, D. Raynaud, J. Jouzel, H. Fischer, K. Kawamura, and T.F. Stocker. 2008. High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 453:379–382. www.ncdc.noaa.gov/paleo/pubs/luethi2008/luethi2008.html.
Law Dome, Antarctica, 75-year smoothed: approximately 1010 CE to 1975 CE
Etheridge, D.M., L.P. Steele, R.L. Langenfelds, R.J. Francey, J.-M. Barnola, and V.I. Morgan. 1998. Historical CO2 records from the Law Dome DE08, DE08-2, and DSS ice cores. In: Trends: A compendium of data on global change. Oak Ridge, TN: U.S. Department of Energy. Accessed September 14, 2005. http://cdiac.ornl.gov/trends/co2/lawdome.html.
Siple Station, Antarctica: approximately 1744 CE to 1953 CE
Neftel, A., H. Friedli, E. Moor, H. Lötscher, H. Oeschger, U. Siegenthaler, and B. Stauffer. 1994. Historical carbon dioxide record from the Siple Station ice core. In: Trends: A compendium of data on global change. Oak Ridge, TN: U.S. Department of Energy. Accessed September 14, 2005. http://cdiac.ornl.gov/trends/co2/siple.html.
Mauna Loa, Hawaii: 1959 CE to 2015 CE
NOAA (National Oceanic and Atmospheric Administration). 2016. Annual mean carbon dioxide concentrations for Mauna Loa, Hawaii. Accessed April 14, 2016. ftp://ftp.cmdl.noaa.gov/products/trends/co2/co2_annmean_mlo.txt.
Barrow, Alaska: 1974 CE to 2014 CE
Cape Matatula, American Samoa: 1976 CE to 2014 CE
South Pole, Antarctica: 1976 CE to 2014 CE
NOAA (National Oceanic and Atmospheric Administration). 2016. Monthly mean carbon dioxide concentrations for Barrow, Alaska; Cape Matatula, American Samoa; and the South Pole. Accessed April 14, 2016. ftp://ftp.cmdl.noaa.gov/data/trace_gases/co2/in-situ/surface.
Cape Grim, Australia: 1992 CE to 2006 CE
Shetland Islands, Scotland: 1993 CE to 2002 CE
Steele, L.P., P.B. Krummel, and R.L. Langenfelds. 2007. Atmospheric CO2 concentrations (ppmv) derived from flask air samples collected at Cape Grim, Australia, and Shetland Islands, Scotland. Commonwealth Scientific and Industrial Research Organisation. Accessed January 20, 2009. http://cdiac.esd.ornl.gov/ftp/trends/co2/csiro.
Lampedusa Island, Italy: 1993 CE to 2000 CE
Chamard, P., L. Ciattaglia, A. di Sarra, and F. Monteleone. 2001. Atmospheric carbon dioxide record from flask measurements at Lampedusa Island. In: Trends: A compendium of data on global change. Oak Ridge, TN: U.S. Department of Energy. Accessed September 14, 2005. http://cdiac.ornl.gov/trends/co2/lampis.html.
Figure 2
EPICA Dome C, Antarctica: approximately 797,446 BCE to 1937 CE
Loulergue, L., A. Schilt, R. Spahni, V. Masson-Delmotte, T. Blunier, B. Lemieux, J.-M. Barnola, D. Raynaud, T.F. Stocker, and J. Chappellaz. 2008. Orbital and millennial-scale features of atmospheric CH4 over the past 800,000 years. Nature 453:383–386. www.ncdc.noaa.gov/paleo/pubs/loulergue2008/loulergue2008.html.
Law Dome, Antarctica: approximately 1008 CE to 1980 CE
Etheridge, D.M., L.P. Steele, R.J. Francey, and R.L. Langenfelds. 2002. Historic CH4 records from Antarctic and Greenland ice cores, Antarctic firn data, and archived air samples from Cape Grim, Tasmania. In: Trends: A compendium of data on global change. Oak Ridge, TN: U.S. Department of Energy. Accessed September 13, 2005. http://cdiac.ornl.gov/trends/atm_meth/lawdome_meth.html.
Cape Grim, Australia: 1985 CE to 2015 CE
NOAA (National Oceanic and Atmospheric Administration). 2016. Monthly mean CH4 concentrations for Cape Grim, Australia. Accessed July 16, 2016. ftp://ftp.cmdl.noaa.gov/data/trace_gases/ch4/flask/surface/ch4_cgo_surface-flask_1_ccgg_month.txt.
Mauna Loa, Hawaii: 1984 CE to 2015 CE
NOAA (National Oceanic and Atmospheric Administration). 2016. Monthly mean CH4 concentrations for Mauna Loa, Hawaii. Accessed July 16, 2016. ftp://ftp.cmdl.noaa.gov/data/trace_gases/ch4/flask/surface/ch4_mlo_surface-flask_1_ccgg_month.txt.
Shetland Islands, Scotland: 1993 CE to 2001 CE
Steele, L.P., P.B. Krummel, and R.L. Langenfelds. 2002. Atmospheric methane record from Shetland Islands, Scotland (October 2002 version). In: Trends: A compendium of data on global change. Oak Ridge, TN: U.S. Department of Energy. Accessed September 13, 2005. http://cdiac.esd.ornl.gov/trends/atm_meth/csiro/csiro-shetlandch4.html.
Figure 3
EPICA Dome C, Antarctica: approximately 796,475 BCE to 1937 CE
Schilt, A., M. Baumgartner, T. Blunier, J. Schwander, R. Spahni, H. Fischer, and T.F. Stocker. 2010. Glacial-interglacial and millennial scale variations in the atmospheric nitrous oxide concentration during the last 800,000 years. Quaternary Sci. Rev. 29:182–192. ftp://ftp.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/epica_domec/edc-n2o-2010-800k.txt.
Antarctica: approximately 1903 CE to 1976 CE
Battle, M., M. Bender, T. Sowers, P. Tans, J. Butler, J. Elkins, J. Ellis, T. Conway, N. Zhang, P. Lang, and A. Clarke. 1996. Atmospheric gas concentrations over the past century measured in air from firn at the South Pole. Nature 383:231–235. ftp://daac.ornl.gov/data/global_climate/global_N_cycle/data/global_N_perturbations.txt.
Cape Grim, Australia: 1979 CE to 2013 CE
AGAGE (Advanced Global Atmospheric Gases Experiment). 2015. Monthly mean N2O concentrations for Cape Grim, Australia. Accessed June 5, 2015. http://ds.data.jma.go.jp/gmd/wdcgg/cgi-bin/wdcgg/catalogue.cgi.
South Pole, Antarctica: 1998 CE to 2015 CE
Barrow, Alaska: 1999 CE to 2015 CE
Mauna Loa, Hawaii: 2000 CE to 2015 CE
NOAA (National Oceanic and Atmospheric Administration). 2016. Monthly mean N2O concentrations for Barrow, Alaska; Mauna Loa, Hawaii; and the South Pole. Accessed June 8, 2016. www.esrl.noaa.gov/gmd/hats/insitu/cats/cats_conc.html.









