Clean Air Markets - Monitoring Surface Water Chemistry
Surface water chemistry is a direct indicator of the effects of acid rain on water bodies. Networks that monitor surface water chemistry over long time periods provide valuable information on aquatic ecosystem health and how water bodies respond to changes in acid-causing emissions. EPA oversees two programs that track changes in surface water chemistry in response to changing air emissions and acid deposition: the Long-Term Monitoring (LTM) program and the Temporally Integrated Monitoring of Ecosystems (TIME) program.
The goal of these programs is to track whether the Clean Air Act Amendments (CAAA) (Plain English Guide to the Clean Air Act) have been effective in reducing the acidity of surface waters in New England, the Northern Adirondack Mountains, Appalachian Plateau, and the Central Appalachians.
What are Acidified Surface Waters?
Acid precipitation (commonly known as acid rain) primarily affects sensitive bodies of water, which are located in watersheds whose soils have a limited ability to neutralize acidic compounds (called “buffering capacity”). Acidification is a result of acidic deposition and the soil’s capacity to buffer what is deposited. As water moves through and over the soil, it picks up the acid deposited and transfers it into lakes and streams. As lakes and streams become more acidic, the numbers and types of fish and other aquatic plants and animals that live in these waters decrease. Learn more about acidification.
Data and Information:
- Interactive Map of LTM Sites Using Google Earth (KMZ)(1 pg, 5 MB, 12/18/2008) — View site information and time series plots of SO4, NO3 and ANC for each lake or stream. Learn about KMZ files.
- List of Related Publications
EPA oversees the LTM and TIME monitoring programs to track the status of acidified and acid-sensitive waters. These programs measure critical chemical variables which provide insights into many measures of ecosystem health. The LTM and TIME programs are complementary monitoring networks used to better understand long-term trends in acidification and recovery in the Northeastern and Mid-Atlantic United States. Both programs are operated with numerous collaborators in state agencies, academic institutions and other federal agencies.
Long Term Monitoring Project (LTM)
The primary objective of the LTM program is to detect long-term trends in acid/base status of lakes and streams across a gradient of acidic deposition. LTM consists of a site network of lakes and streams that are particularly sensitive to acidity due in part to their underlying geology. Most site records date back to the mid 1980s or earlier. Sites are sampled 3 to 15 times per year with a higher proportion of sampling conducted in the spring to coincide with higher rates of runoff (e.g., snow melt) and higher stream flows because the aquatic effects of acidic deposition often exhibit seasonality (e.g., causing harm in the winter or spring when the more sensitive life stages of some fish species are present). LTM water chemistry data are used to characterize how the most sensitive aquatic systems in each region are responding to changing acid deposition, as well as providing information on seasonal chemistry and episodic acidification.
Temporally Integrated Monitoring of Ecosystems (TIME)
TIME was developed as a special study within EPA's Environmental Monitoring and Assessment Program (EMAP)-Surface Waters to track long term trends in the acid relevant chemistry of specific classes of acid-sensitive lakes and streams. The TIME monitoring program consists of probability surveys designed to answer questions like: “how many acidic lakes are there?" and "what is the trend in the number or proportion of lakes which are acidic?” These can be answered for specific types of lakes (e.g., seepage vs. drainage lakes) or for specific regions (e.g., Adirondacks vs. New England uplands). TIME is statistically based, thereby allowing regional population estimates of acid sensitivity status to be developed. TIME consists of a synoptic survey of 74 acid-sensitive lakes in the Northeast and a separate synoptic survey of 58 acid-sensitive streams in the Mid-Atlantic. Sites are sampled annually in the late summer (lakes) and spring (streams) and the regional estimates they generate are specific to that time period.
TIME and LTM Sampling Regions and Cooperators
Adirondack Mountains
- Participation —sample collection and laboratory analysis for seasonal or monthly samples from 52 LTM lakes and the collection of annual samples for 43 TIME lakes in the Adirondack Mountains of New York
- Cooperator(s) — Adirondack Lake Survey Corporation Exit, New York State Energy Research and Development Authority Exit, New York State Department of Environmental Conservation Exit
- Period of Record — mid 1980s through present
New England
Vermont
- Participation — sampling and laboratory analysis during spring runoff on 7 lake outlets and open water sampling during the spring, summer and fall from 12 LTM lakes
- Cooperator(s) — Vermont Department of Environmental Conservation Exit
- Period of Record — 1983 through present
Maine
- Participation — sample collection and laboratory analysis for quarterly samples from 15 Maine LTM lakes and the collection of annual samples for 31 TIME New England lakes in Maine, New Hampshire, Massachusetts and Rhode Island, and all the TIME lake laboratory analyses (74, including the Adirondack lakes)
- Cooperator(s) —US Geological Survey, University of New Hampshire Exit, and University of Maine Exit
- Period of Record — 1982 through present
Northern Appalachian Plateau
Catskill Mountains, NY
- Participation — sample collection, laboratory analysis and stream gauging for monthly samples from 4 Catskill Mountain streams
- Cooperator(s) — US Geological Survey District Office in Troy, NY
- Period of Record — 1983 through present
Pennsylvania
- Participation — sample collection, laboratory analysis and stream gauging for monthly samples from 5 headwater Pennsylvania streams, and sample collection and laboratory analysis for 24 annual TIME streams in Pennsylvania
- Cooperator(s) —US Geological Survey, Pennslyvania State University Exit
- Period of Record — 1988 through present
Central Appalachians
Virginia
- Participation — sample collection and laboratory analysis for weekly samples at four sites, seasonal samples at 72 sites, sample collection every two hours during episodic high-flow conditions at three sites with continuous discharge gauging in Virginia streams, and 34 annual TIME streams in Virginia, West Virginia and Maryland
- Cooperator(s) —National Park Service, University of Virginia Exit
- Period of Record — 1979 through present
Locations for the TIME and LTM Programs
The TIME and LTM programs are designed to track changes in surface water chemistry in the four regions shown below, known to be sensitive to acid rain: New England, the Adirondack Mountains, the Northern Appalachian Plateau, and the central Appalachians.
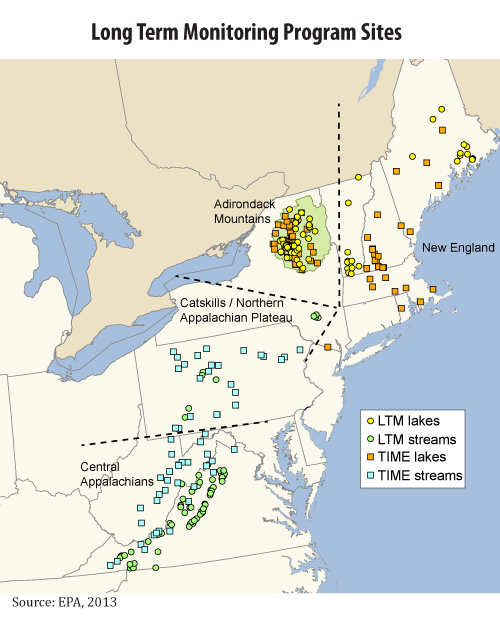
The TIME and LTM networks provide information on the chemistry of lakes and streams to indicate how water bodies are responding to changes in SO2 and NOx emissions.
| Region | Water Bodies Covered | % of Sites with Improving Sulfate Trend | % of Sites with Improving Nitrate Trend | % of Sites with Improving ANC Trend | % of Sites with Improving Base Cations Trend | % of Sites with Increasing DOC Trend |
|---|---|---|---|---|---|---|
| Adirondack Mountains | 50 lakes in NY | 100% | 54% | 76% | 88% | 62% (29 sites) |
| New England | 26 lakes in ME and VT | 100% | 18% | 43% | 74% | 39% (13 sites) |
| Catskills/N. Appalachian Plateau | 9 streams in NY and PA | 80% | 40% | 58% | 90% | 0% (9 sites) |
| Central Appalachians | 66 streams in VA | 15% | 58% | 15% | 14% | NA |
- Trends are determined by multivariate Mann-Kendall tests
- Trends are significant at the 95 percent confidence interval (p < 0.05)
- Data for 5 Pennsylvania N. Appalacian Plateau sites is unavailable for 2012
- DOC was only examined in low-ANC waterbodies (ANC less than 25 μeq/L)
- DOC is not currently measured in Central Appalachian streams
Source: EPA, 2014
This table shows regional trends in acidification from 1990 (before implementation of the Acid Rain Program) to 2012 in lakes and streams throughout the LTM program. Acidified drainage water mobilizes toxic forms of aluminum from soils and clays, harming fish and wildlife. Five chemical indicators of aquatic ecosystem response to emission changes are presented: trends in sulfate and nitrate anions, base cations, acid neutralizing capacity (ANC), and dissolved organic carbon (DOC). These indicators provide information regarding the surface water sensitivity to acidification and the degree of impact on the aquatic ecosystem. Trends in these measured chemical indicators in drainage waters allow for the determination of whether the water bodies are improving and heading towards recovery of if they are still acidifying. The following is a description of each indicator represented in the table:
Sulfate is the primary anion in most acid-sensitive waters and has the potential to acidify drainage waters and leach base cations and toxic forms of aluminum from the soils.
Nitrate has the same potential as sulfate to acidify drainage waters. However, nitrogen is an important nutrient for plant growth and a large portion of nitrogen inputs from deposition are quickly incorporated into plants as organic nitrogen, leaving less leaching of nitrate into surface waters. Water nitrate concentrations are often variable and reductions in nitrogen deposition are slow to be reflected in long term trends.
Base cations is the sum of the positively charged calcium, magnesium, sodium and potassium ions in soils and surface waters that buffer both sulfate and nitrate anions, thereby preventing surface water acidification. Base cation availability is largely a function of underlying geology and soil age, such that young soils of cation-rich bedrock will tend to have a greater buffering capacity.
Acid Neutralizing Capacity (ANC) is a measure of overall buffering capacity against acidification, and indicates the ability to neutralize strong acids that enter aquatic systems. When ANC is low, and especially when it is negative, stream water pH is also low (less than pH 6, commonly less than pH 5), and may be harmful to fish and other aquatic organisms essential for a healthy aquatic ecosystem. Recovery of an aquatic ecosystem is indicated by increasing trends in ANC and base cations and decreasing trends in sulfate and nitrate concentrations.
Dissolved organic carbon (DOC) is essentially dissolved organic material that is an important part of the acid-base chemistry of most freshwater systems (particular low-ANC waterbodies) because it can assist in neutralizing strong acids. A host of factors control DOC concentrations in surface waters and increases can be indicative of reduced acidification and/or a sign of increased decomposition of organic matter in the watershed.
